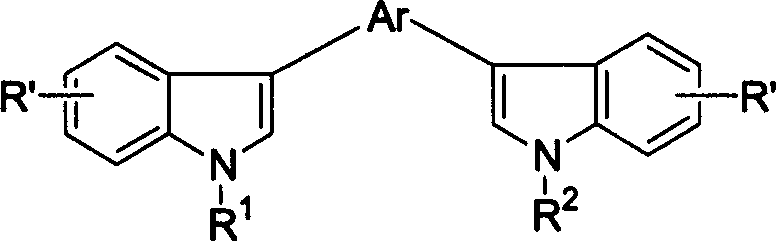
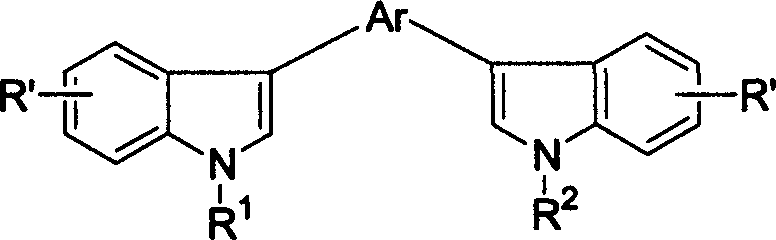
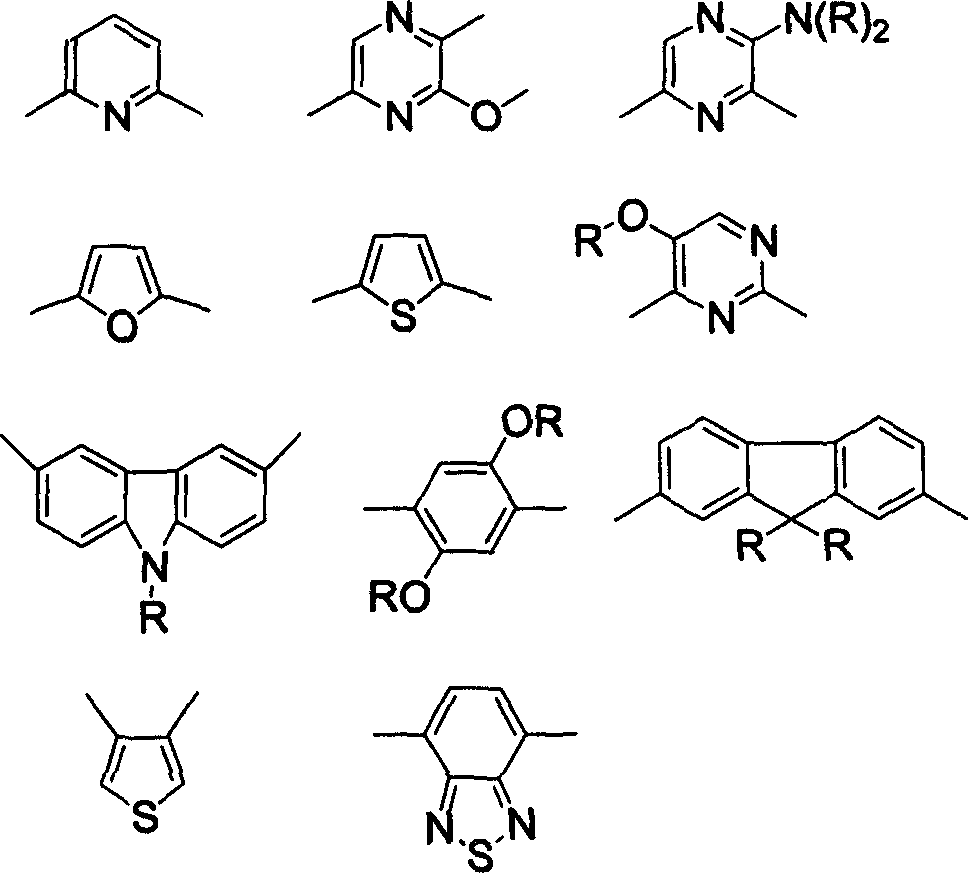
Compound of biindole heterocycles, preparation method and application in use for organic electroluminescence material
A compound, indole technology, applied in the directions of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of complex synthesis route, high material cost, low stability, etc., and achieves simple preparation process, good optical performance, easy to use. The effect of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 3-methoxyl group-2, the synthesis of 5-bis(N-benzyl-3'-indole)pyrazine
[0032] Under argon protection, 825 mg (3 mmol) N-tert-butyldimethylsilyl-3-indole borate ethylene glycol ester (according to Journal of Organic Chemistry, 1994, 59, 10-11 prepared by the reported method), 268 mg (1 mmol) 3-methoxy-2,5-dibromopyrazine and 116 mg (0.1 mmol) Pd (PPh 3 ) 4 , after replacing the gas in the reaction bottle with an inert gas, add 20mL of toluene and 20mL of ethylene glycol dimethyl ether, stir well, and then add 3mL of 2M sodium carbonate aqueous solution. The mixture was heated to 80-100°C and stirred at this temperature for about 24 hours. After cooling to room temperature, add 30 mL of water. Extracted with ethyl acetate (2×50 mL); the organic phases were combined, washed with saturated brine and dried over anhydrous sodium sulfate, and the organic solvent was evaporated under reduced pressure. The residue was dissolved in tetrahydrofuran, added TBAF (tet...
Embodiment 2
[0035] Example 2 Synthesis of 2-dimethylamino-3,5-bis(N-benzyl-3'-indole)-pyrazine
[0036] Under the protection of argon, 3.85g (14mmol) N-tert-butyldimethylsilyl-3-indole ethylene glycol borate, 1.267g (4.6mmol) 3-methoxy- 2,5-Dibromopyrazine, 531mg (0.46mmol) Pd(PPh 3 ) 4 . After the reaction flask was replaced with an inert gas, 40 mL of toluene and 40 mL of ethylene glycol dimethyl ether were added, and after uniform stirring, 14 mL of 2M sodium carbonate aqueous solution was added. The mixture was heated to 80-100°C and stirred at this temperature for about 24 hours. After the reaction mixture was cooled to room temperature, 50 mL of water was added and extracted with ethyl acetate (2×50 mL). The organic phases were combined, washed with saturated brine and dried over anhydrous sodium sulfate, and the organic solvent was distilled off under reduced pressure. The residue was dissolved in tetrahydrofuran, added TBAF in tetrahydrofuran (1M, 14mL, 14mmol), stirred at ro...
Embodiment 3
[0039] Example 3 Synthesis of 2,5-bis(N-benzyl-3'-indole)-pyrazine
[0040] Under argon protection, 3.3g (12mmol) N-tert-butyldimethylsilyl-3-indole boronic acid, 952mg (4mmol) 2,5-dibromopyrazine, 463mg (0.4mmol) Pd (PPh 3 ) 4 Sequentially add to a 200mL reaction flask, fill the reaction flask with an inert gas, add 50mL of toluene and 50mL of ethylene glycol dimethyl ether, stir well and add 12mL of 2M sodium carbonate aqueous solution. The mixture was heated to 80-100°C and stirred at this temperature for about 24 hours. After cooling to room temperature, 50 mL of water was added and extracted with ethyl acetate (2×50 mL). The organic phases were combined and washed with saturated brine, dried over anhydrous sodium sulfate, and the organic solvent was evaporated under reduced pressure. The residue was dissolved in tetrahydrofuran, added TBAF in tetrahydrofuran (1M, 12mL, 12mmol), stirred at room temperature for 6 hours, added water 20mL, extracted with ethyl acetate (2×3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com