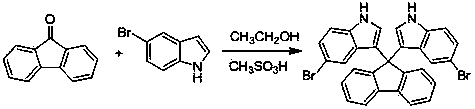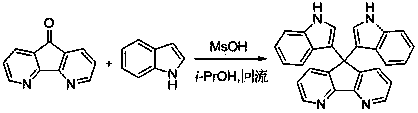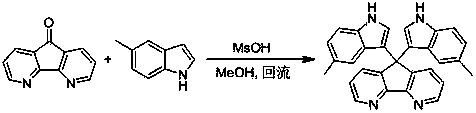Method for preparing bi-indolyl fluorene derivative
A technology for bis-indolyl fluorene and derivatives is applied in the field of preparing bis-indolyl fluorene derivatives, and achieves the effects of mild reaction conditions, few operation steps and high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: This example is the synthesis of 9,9'-bis(7-methyl-3-indolyl)fluorene; using 9-fluorenone and 7-methylindole as raw materials, the reaction formula is as follows:
[0036]
[0037] Implementation steps:
[0038] 0.180 g (1 mmol) of 9-fluorenone, 0.328 g (2.5 mmol) of 7-methylindole and 0.096 g (0.1 mmol) of methanesulfonic acid were placed in a reaction flask, 25 mL of methanol was added, and heated to reflux (reaction solution The temperature is about 65° C.), followed by TLC until the end of the reaction, cooled to room temperature, and suction filtered to obtain a solid. The solid was recrystallized from DMSO to give pure product (74% yield).
[0039] Product data: 1 H NMR (400 MHz, DMSO-d 6 ) δ 10.81 (s, 1H), 7.94 (d, J = 7.5 Hz, 1H), 7.54 (d, J = 7.6 Hz, 1H), 7.36 (t, J = 7.4 Hz, 1H), 7.19 (t, J = 7.4 Hz, 1H), 6.77 (d, J = 6.5 Hz, 2H), 6.70 – 6.58 (m, 2H), 2.40 (s, 3H); 13 C NMR (101 MHz, DMSO-d 6 ) δ 152.91, 139.48, 136.96, 127.67 (d, ...
Embodiment 2
[0040] Example 2: This example is the synthesis of 9,9'-bis(5-bromo-3-indolyl)fluorene; using 9-fluorenone and 5-bromoindole as raw materials, the reaction formula is as follows:
[0041]
[0042] Implementation steps:
[0043] 0.180 grams (1 mmol) of 9-fluorenone, 0.485 grams (2.5 mmol) of 5-bromoindole and 0.096 grams (0.1 mmol) of methanesulfonic acid were placed in a reaction flask, 25 mL of ethanol was added, and heated to reflux (reaction temperature About 78°C), followed by TLC until the end of the reaction, cooled to room temperature, and suction filtered to obtain a solid. The solid was recrystallized from dimethylsulfoxide to obtain pure product (yield 88%).
[0044] Product data: 1 H NMR (400 MHz, DMSO-d 6 ) δ 11.17 (s, 1H), 8.00 (d, J = 7.5 Hz, 1H), 7.49 – 7.37 (m, 2H), 7.35 – 7.24 (m, 2H), 7.10 (dd, J = 8.6, 1.5 Hz, 1H), 6.98 (d, J = 2.2 Hz, 1H), 6.74 (s, 1H); 13 C NMR (101 MHz, DMSO-d 6 ) δ 152.02, 139.41, 136.14, 128.08, 127.84, 125.86, 125.57, 123...
Embodiment 3
[0045]Example 3: This example is the synthesis of 9,9'-bis(2-methyl-3-indolyl)fluorene; using 9-fluorenone and 2-methylindole as raw materials, the reaction formula is as follows:
[0046]
[0047] Implementation steps:
[0048] 0.180 grams (1 mmol) of 9-fluorenone, 0.328 grams (2.5 mmol) of 2-methylindole and 0.096 grams (0.1 mmol) of methanesulfonic acid were placed in a reaction flask, 25 mL of propanol was added, heated to reflux (reaction Liquid temperature is about 97°C), TLC tracking until the end of the reaction, cooled to room temperature, and suction filtration to obtain a solid. The solid was recrystallized from dimethyl sulfoxide to obtain pure product (97% yield).
[0049] Product data: 1 H NMR (400 MHz, DMSO-d 6 ) δ 10.71 (s, 1H), 7.91 (d, J = 7.5 Hz, 1H), 7.53 (d, J = 7.6 Hz, 1H), 7.35 (t, J = 7.4 Hz, 1H), 7.16 (t, J = 7.2 Hz, 2H), 6.84 (t, J = 7.5 Hz, 1H), 6.54 (t, J = 7.1 Hz, 2H), 1.73 (s, 3H); 13 C NMR (101 MHz, DMSO-d 6 ) δ 153.50, 139.32...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com