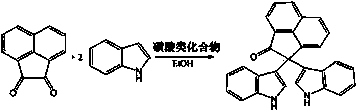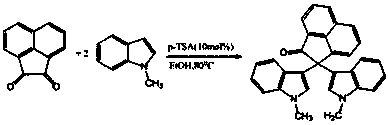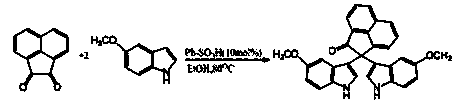2,2-di(1H-indole-3-yl)-2H-acenaphthene-1-ketone compound and preparation method thereof
A technology of ketone compounds and compounds, which is applied in the field of organic chemical synthesis, can solve problems such as cumbersome operating procedures, unfavorable industrial production, and low yield, and achieve the effects of simple operating procedures, low equipment requirements, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044] In a 50mL round-bottom flask, add 91mg acenaphthenequinone (MW=182, 0.5mmol), 117mg indole (MW=117, 1.0mmol), 11.6mg camphorsulfonic acid (CSA) (MW=232, 0.05mmol), 5 mL of absolute ethanol, the reaction solution was stirred at 80°C under reflux for 15 minutes (the reaction progress was monitored by TLC, developing solvent: 60-90 petroleum ether: ethyl acetate = 2:1, volume ratio). After the reaction was completed, the reaction solution was cooled to room temperature, 5 mL of distilled water was added, a yellow solid powder was precipitated, suction filtered, washed twice with 1 mL of cold absolute ethanol, and vacuum-dried to obtain 197.0 mg of the product with a yield of 99%. The purity measured by HPLC was: 99.5%. 1 H NMR (500 MHz, DMSO -d 6 ): δ 10.98 (s, 2H, NH), 8.37 (d, J = 8.0 Hz, 1H), 8.04–7.97 (m, 2H), 7.91–7.88 (m, 1H), 7.71–7.68 (m, 1H), 7.55 (d, J = 6.7 Hz, 1H), 7.35 (d, J = 8.0 Hz, 2H), 7.02–6.99 (m, 4H), 6.85 (s, 2H), 6.74 (t, J = 7.4...
Embodiment 3
[0048] Comparative example, other operations are the same as in Example 1. The reaction solution was stirred at 40° C. for 15 minutes, and processed according to Example 1 to obtain 189.1 mg of the product with a yield of 95%.
Embodiment 4
[0050] Comparative example, other operations are the same as in Example 1. The reaction solution was stirred at 60° C. for 15 minutes, and processed according to Example 1 to obtain 193.0 mg of the product with a yield of 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com