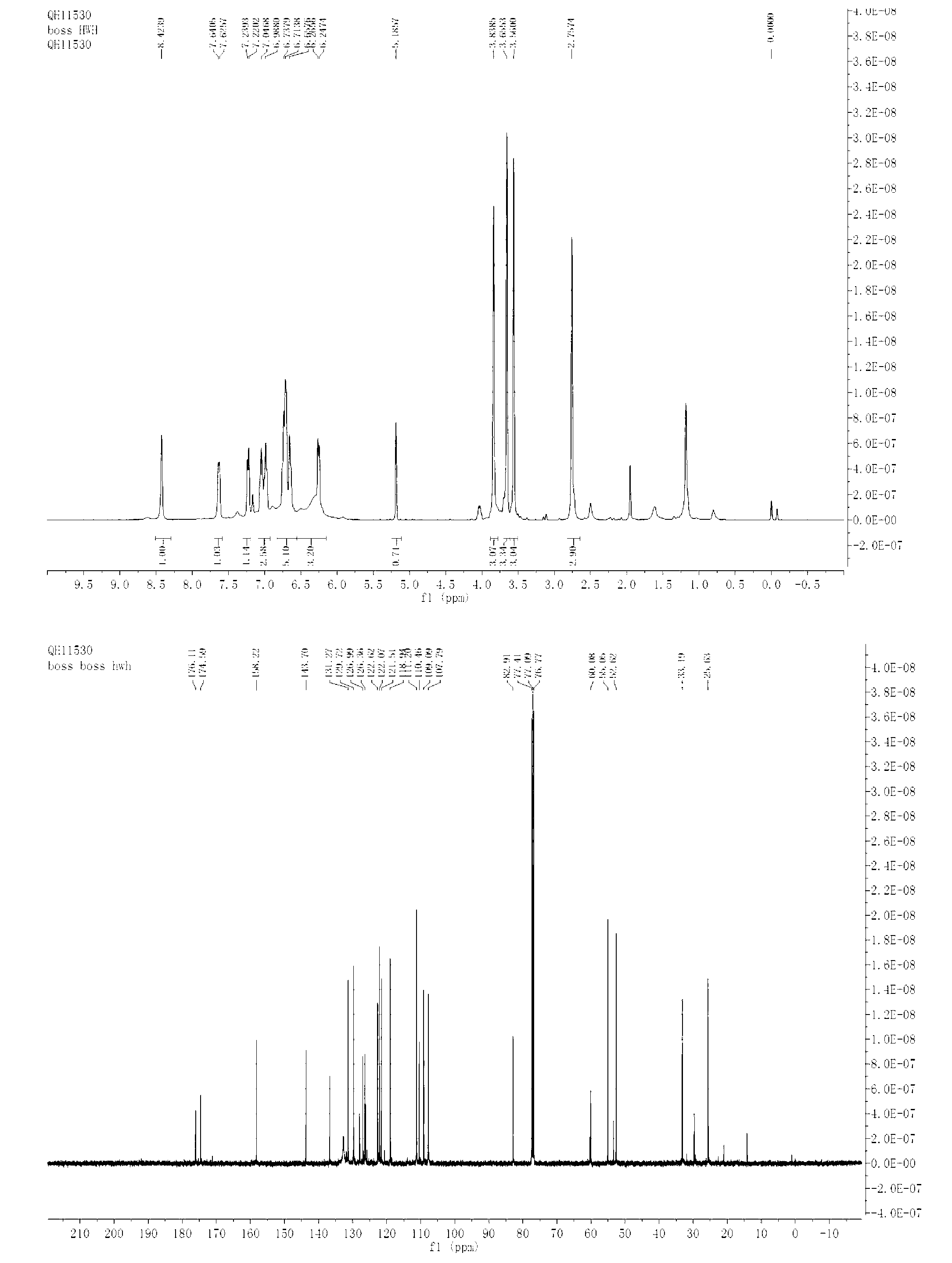Bisindole alkaloid derivative, and synthesis method and application thereof
A technology of indole alkaloids and indole derivatives, which is applied in the direction of drug combination, organic chemistry, anti-infective drugs, etc., can solve the problems of cumbersome operation, low yield, high cost, etc., and achieve simple and safe operation and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
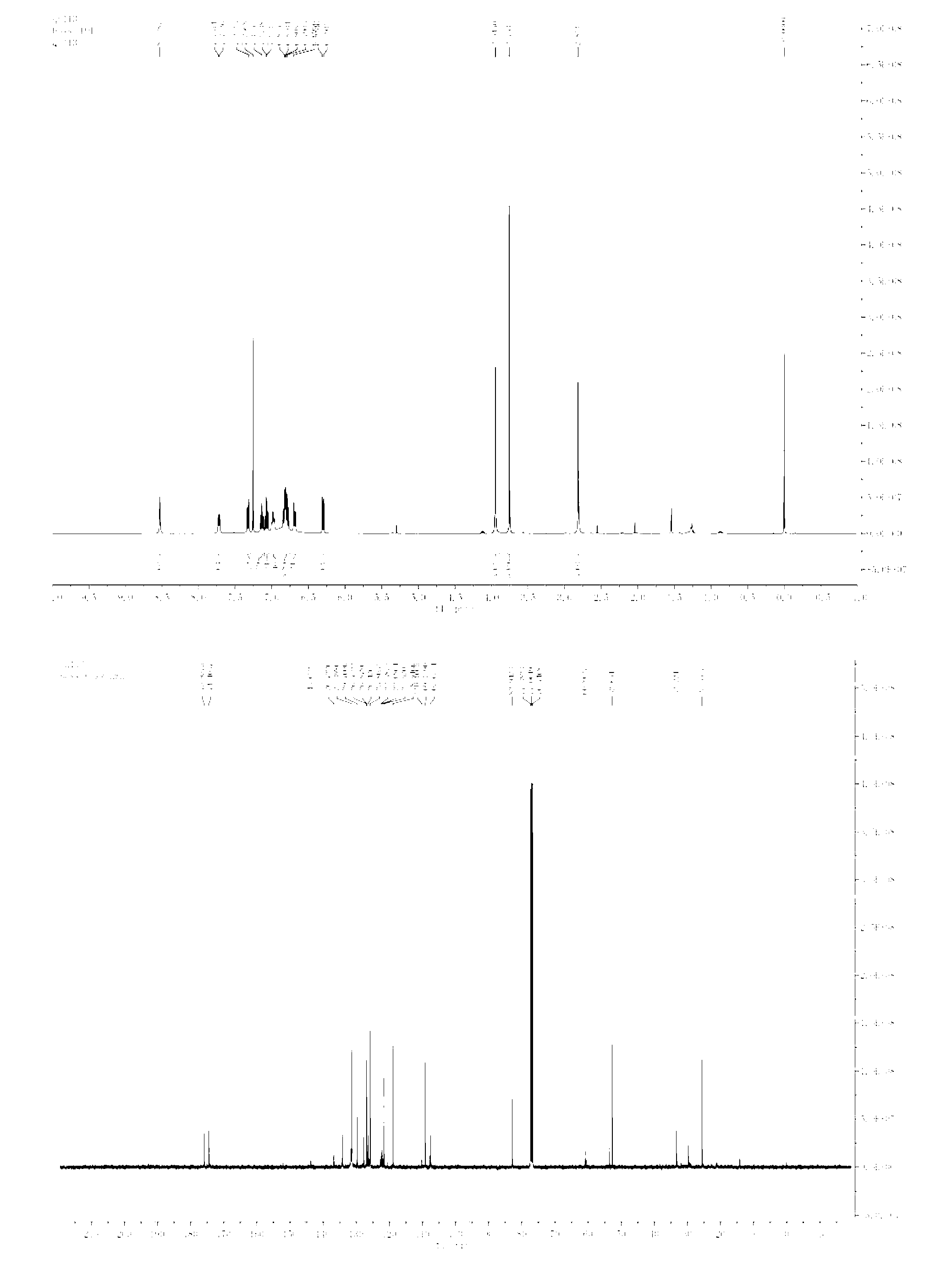
[0031] N-methylindole (0.3mmol), Rh 2 (OAc) 4 (0.0025mmol), N-methyl indigo (0.3mmol) red was dissolved in toluene (1ml), then, phenyldiazoacetate (0.25mmol,) dissolved in toluene (1.0ml) in 1 hour Add it dropwise into the reaction system, and the reaction system is at 0°C. After the dropwise addition, stir for 1 hour, and remove the solvent by rotary evaporation under reduced pressure to obtain a crude product, whose structure is shown in formula (2-1). The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:20~1:3) to obtain a pure product. Yield 81%, d.r > 20:1. nuclear magnetic resonance 1 H NMR, 13 C NMR spectrum as figure 1 As shown, the product 2-1 1 H NMR (400MHz, CDCl3) δ (ppm) 8.53 (s, 1H), 7.72 (d, J=6.7Hz, 1H), 7.30-7.32 (m, 1H), 7.04-7.15 (m, 3H), 6.98 ( m, 2H), 6.77-6.84(m, 4H), 6.68-6.70(m, 1H), 6.69(d, J=7.6Hz, 1H), 3.93(s, 3H), 3.74(s, 3H), 2.81 (s, 3H); 13 C NMR(100Hz,CDCl3)δ(ppm)175.96,174.40,143.65,131....
Embodiment 2
[0033]
[0034] N-methylindole (0.3mmol), N-methylisatin (0.3mmol), Rh 2 (OAc) 4(0.0025mmol), dissolved in dichloromethane (1ml), then, methyl p-methoxyphenyldiazoacetate (0.25mmol,) dissolved in dichloromethane (1.0ml) was added dropwise in 1 hour Into the reaction system, the reaction system was at 50°C, after the dropwise addition was completed, stirred for 1 hour, and the solvent was removed by rotary evaporation under reduced pressure to obtain a crude product, the structure of which was shown in formula (2-2). The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:20~1:10) to obtain a pure product. Yield 73%, d.r > 20:1. nuclear magnetic resonance 1 H NMR, 13 C NMR spectrum as figure 2 As shown, the product 2-2 1 H NMR (400MHz, CDCl3) δ (ppm) 8.42 (s, 1H), 7.63 (d, J = 5.9Hz, 1H), 7.22-7.24 (m, 1H), 6.99-7.05 (m, 3H), 6.66- 6.74(m, 5H), 6.24-6.60(m, 3H), 3.84(s, 3H), 3.66(s, 3H), 3.56(s, 3H), 2.75(s, 3H); 13 C NMR(100Hz,CDCl...
Embodiment 3
[0036]
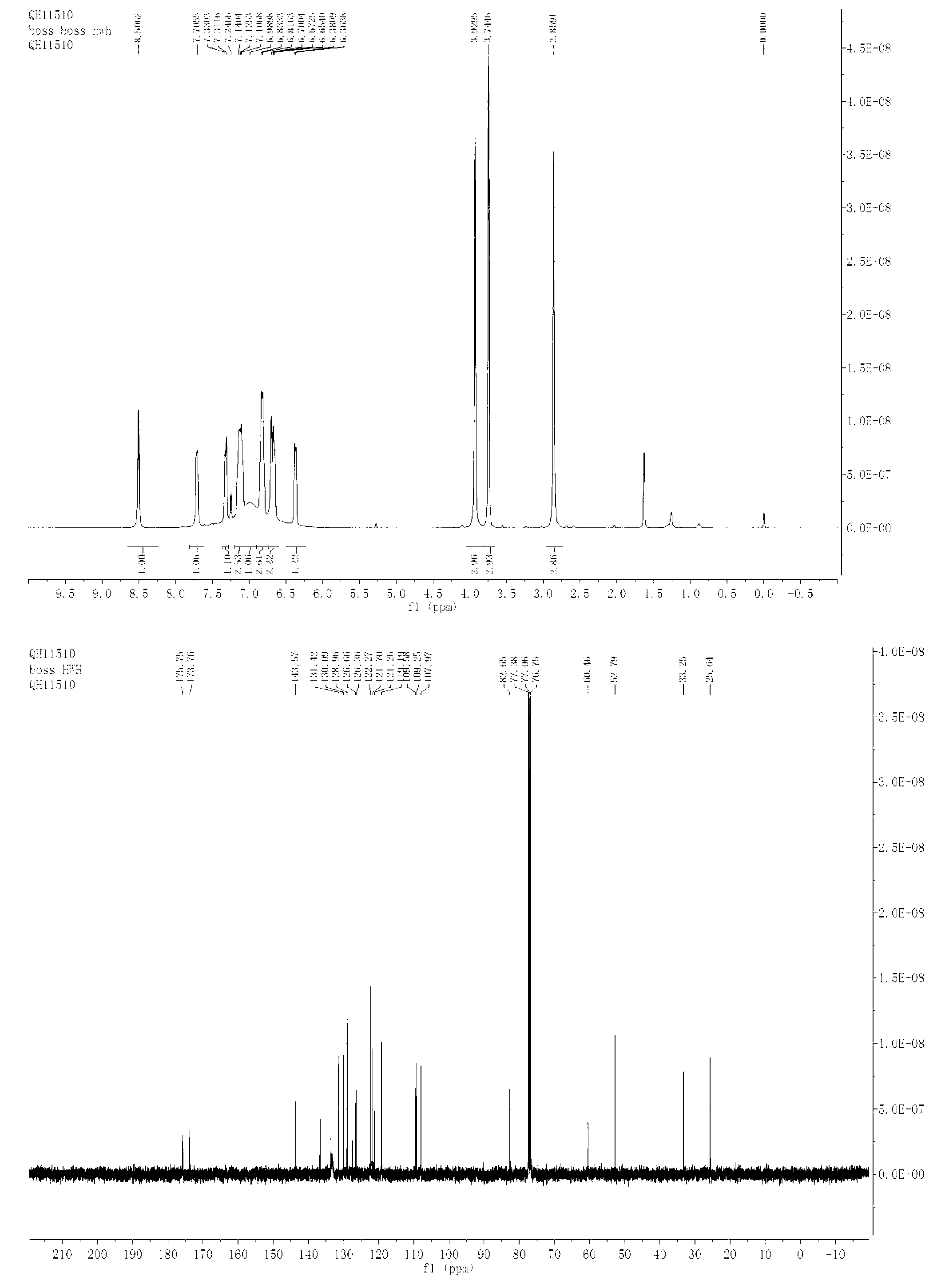
[0037] N-methylindole (0.3mmol), N-methylisatin (0.3mmol), Rh 2 (OAc) 4 (0.0025mmol) was dissolved in xylene (1ml), then, 4-bromophenyldiazoacetic acid methyl ester (0.25mmol,) dissolved in xylene (1.0ml) was added dropwise to the reaction system within 1 hour , the reaction system was at -10°C, after the dropwise addition was completed, it was stirred for 1 hour, and the solvent was removed by rotary evaporation under reduced pressure to obtain a crude product, the structure of which was shown in formula (2-3). The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:20~1:5) to obtain a pure product. Yield 82%, d.r > 20:1. nuclear magnetic resonance 1 H NMR, 13 C NMR spectrum as image 3 As shown, the products 2-3 1 H NMR (400MHz, CDCl 3 )δ (ppm) 8.51 (s, 1H), 7.71 (m, 1H), 7.31-7.33 (m, 1H), 7.11-7.13 (m, 3H), 6.98 (m, 2H), 6.82-6.83 (m, 2H), 6.65-6.67(m, 2H), 6.37(d, J=6.8Hz, 1H), 3.93(s, 3H), 3.74(s, 3H), 2.86(s, 3H); 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com