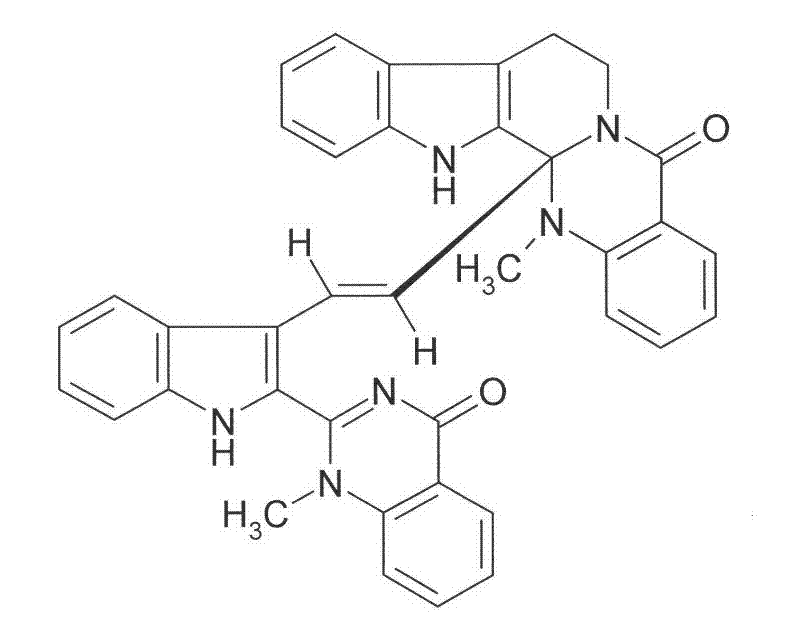
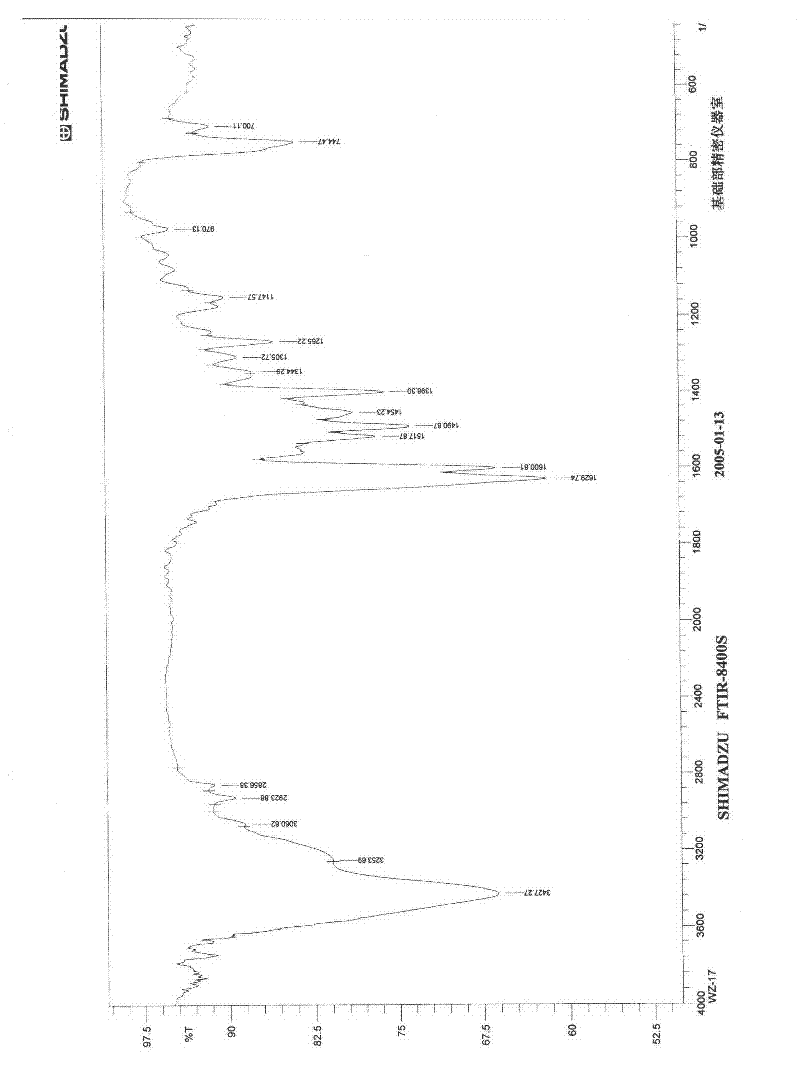
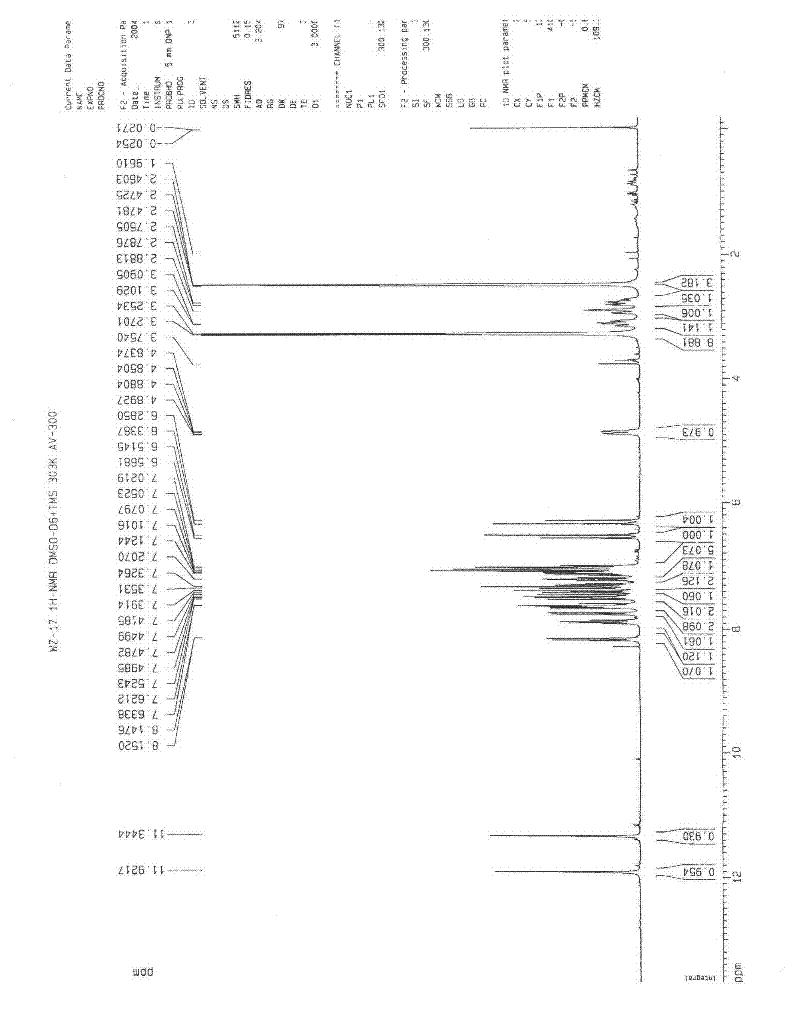
Application of diindoloquinazoline alkaloid in preparation of antitumor drugs and antifungal drugs
A technology of bisindolequinazoline and indolequinazoline, which can be applied in the directions of antitumor drugs, antifungal agents, drug combinations, etc., can solve the problems of high toxicity or irritation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1. Isolation and identification of compound I from raw Evodia rutaecarpa
[0030] Raw Evodia rutaecarpa 4Kg, first take out 200g and use a volatile oil extractor to extract about 2ml of Evodia rutaecarpa volatile oil, and prepare it for GC-MS. The dregs and the remaining raw Evodia rutaecarpa were combined and extracted 3 times with 80% ethanol under reflux, each time for 3 hours, the alcohol extract was combined, concentrated under reduced pressure to obtain about 800 g of thick extract, dissolved in water to a suspension, and successively washed with petroleum ether (60 -90°C), chloroform, ethyl acetate and water-saturated n-butanol were extracted, concentrated into extract respectively to obtain petroleum ether fraction 14g, chloroform fraction 209g, ethyl acetate fraction 26g, n-butanol fraction 210g, and water fraction 316g. The chloroform part was repeatedly passed through silica gel normal pressure (LC), low pressure (LPLC) and medium pressure column chro...
Embodiment 2
[0043] Example 2. Isolation and Identification of Compound I from Glycyrrhizae Evodiaecarpa
[0044] Evodia rutaecarpa 9.5Kg was prepared from sugar, cold soaked in petroleum ether for 7 days to obtain 67g of petroleum ether extract; the dregs after degreasing were reflux extracted with 80% ethanol for 3 times, each time for 3 hours, combined with alcohol extracts, concentrated under reduced pressure to obtain The thick extract is about 1.5Kg; add 2% acid water and knead to dissolve to the suspension, and extract the acid water with ethyl acetate to obtain 263g of ethyl acetate extract; the extracted acid water is alkalized to pH 9-10 by adding ammonia water , extracted with chloroform to obtain 126g of chloroform extract with more concentrated total alkaloids; finally the alkaline water was adjusted to neutral and extracted with n-butanol to obtain 293g of n-butanol extract. The chloroform extract was repeatedly passed through silica gel normal pressure (LC), low pressure (LP...
Embodiment 3
[0045] Example 3. Anti-tumor activity evaluation experiment of compound 1 by zebrafish method
[0046] 1. Research materials:
[0047] 1.1 Zebrafish embryos
[0048] 1.2 Screening Compounds
[0049] Hydroxyevodiamine, Evodiamine, Evodiamine, Evodiamide I, Evodiamine
[0050] 2. Experimental method
[0051] 2.1 Zebrafish model
[0052] 2.1.1 Model Basis
[0053] The occurrence of tumor is a complex and multi-link process of transforming normal cells into cancer cells. The size of the tumor exceeds 0.2-2.0 (about 10 5 ~10 6 cells), it begins to build its own blood supply system, that is, new angiogenesis. Angiogenesis is a process in which new blood vessels grow from lateral sprouts protruding from the existing vascular system, and its main feature is the proliferation of endothelial cells (EC) lining the inside of new blood vessels. This endothelial cell proliferates extremely rapidly, far exceeding other normal tissues, and this unique phenotype of endothelial cells is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tumor inhibition rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com