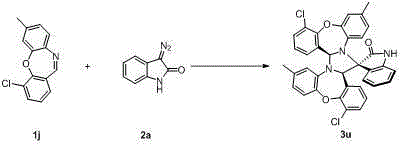
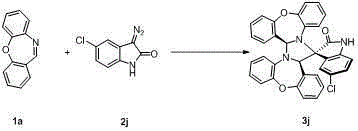
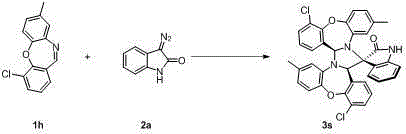
Spiro oxindole imidazolinyl oxazepine compound and synthesis method thereof
A technology of spirocyclic oxindole imidazoline and oxazepine, which is applied in the field of synthesis of spirocyclic oxindole imidazoline compounds and achieves excellent diastereoselectivity, high yield and good chemoselectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Add p-toluenesulfonic acid monohydrate (0.95mg, 0.005mmol) and 1a (43.1 mg, 0.22 mmol), 2a (15.9mg, 0.1mmol), add 2mL tetrahydrofuran, stir and react at room temperature for 24 hours, the reaction system can be obtained by simple column chromatography (dichloromethane:petroleum ether=2:1) to obtain the target product 3a (Yield: 27.6mg, 53%), white solid, diastereoselectivity>99 / 1.
[0033] Add iron (II) trifluoromethanesulfonate (1.8 mg, 0.005 mmol) and 1a (43.1 mg, 0.22 mmol), 2a (15.9mg, 0.1mmol), add 2mL tetrahydrofuran, stir and react at room temperature for 24 hours, the reaction system can be obtained by simple column chromatography (dichloromethane:petroleum ether=2:1) to obtain the target product 3a (Yield 21.3 mg, 41% yield), white solid, diastereoselectivity >99 / 1.
[0034] P-toluenesulfonic acid monohydrate (1.9mg, 0.01mmol) or iron (II) trifluoromethanesulfonate (3.5mg, 0.01mmol) was added sequentially into the reaction flask as a catalys...
Embodiment 2
[0038]
[0039] Add p-toluenesulfonic acid monohydrate (1.9mg, 0.01mmol) to the reaction flask successively, 1a (43.1 mg, 0.22 mmol), 2b (15.9mg, 0.1mmol), add 2mL tetrahydrofuran, stir and react at room temperature for 24 hours, the reaction system can be obtained by simple column chromatography (dichloromethane:petroleum ether=1:1) to obtain the target product 3a (48.4 mg, 93% yield), white solid, diastereoselectivity >99 / 1.
[0040] Iron (II) trifluoromethanesulfonate (3.5 mg, 0.01 mmol) was successively added into the reaction flask, 1a (43.1 mg, 0.22 mmol), 2b (15.9mg, 0.1mmol), add 2mL tetrahydrofuran, stir and react at room temperature for 24 hours, the reaction system can be obtained by simple column chromatography (dichloromethane:petroleum ether=1:1) to obtain the target product 3a (45.8 mg yield, 88% yield), white solid, diastereoselectivity >99 / 1.
[0041] to product 3b After analysis, the results are as follows: 1 HNMR (400MHz, CDCl3) δ7.42 (dd, J =7.2,0...
Embodiment 3
[0043]
[0044] Add p-toluenesulfonic acid monohydrate (1.9mg, 0.01mmol) to the reaction flask successively, 1a (43.1 mg, 0.22 mmol), 2c (24.9mg, 0.1mmol), add 2mL diethyl ether, stir and react at room temperature for 24 hours, the reaction system can be obtained by simple column chromatography (eluent: dichloromethane:petroleum ether=1:1) to obtain the target product 3c (Yield: 47.9 mg, 92%), white solid, diastereoselectivity >99 / 1.
[0045] Iron (II) trifluoromethanesulfonate (3.5 mg, 0.01 mmol) was successively added into the reaction flask, 1a (43.1 mg, 0.22 mmol), 2c (24.9mg, 0.1mmol), add 2mL tetrahydrofuran, stir and react at room temperature for 24 hours, the reaction system can be obtained by simple column chromatography (dichloromethane: petroleum ether = 1:1 as the eluent) to obtain the target product 3c (Yield: 46.3 mg, 89%), white solid, diastereoselectivity >99 / 1.
[0046] to product 3c After analysis, the results are as follows: 1 HNMR (400MHz, CDCl 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com