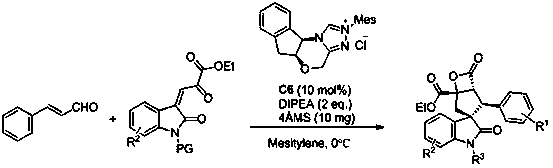
Synthesis method of spiro hydroxyindolocyclopentane beta-exo fat compound
A technology of spirocyclic oxindole cyclopentane and synthesis method, which is applied in the field of synthesis of spirocyclic oxindole compounds, can solve problems such as being difficult to obtain, and achieves easy operation, excellent diastereoselectivity, and enantioselectivity. good effect of sex and diastereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] The chemical structural formula of nitrogen heterocyclic carbene is as follows:
[0031]
[0032] Add nitrogen heterocyclic carbene (3.7 mg, 0.01 mmol) as a catalyst, and 1a (19.8 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol), 4Å molecular sieves (10 mg) in sequence in the reaction flask, add 0.5 mL of Toluene was reacted at 0°C for 24 hours. The reaction system was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:5) to obtain the target product 3a (21.1 mg) as a white solid. The rate was 54%, >99 / 1 dr, 91% ee; antibacterial drugs could be prepared.
[0033] Add nitrogen heterocyclic carbene (1.85 mg, 0.005 mmol) as a catalyst, and 1a (19.8 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol), 4Å molecular sieves (5 mg) in turn into the reaction flask, add 0.5 mL mesitylene , reacted at 0°C for 24 hours, and the reaction system was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:5) to obtain the target product ...
Embodiment 2
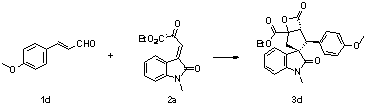
[0037]
[0038] Add nitrogen heterocyclic carbene (3.7 mg, 0.01 mmol) as a catalyst, and 1b (24.3 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol), 4Å molecular sieves (10 mg) in sequence in the reaction flask, add 0.5 mL of Toluene was reacted at 0°C for 24 hours, and the reaction system was subjected to simple column chromatography (eluent: ethyl acetate: petroleum ether = 1:5) to obtain the target product 3b (23.9 mg) as a white solid. The rate was 57%, >99 / 1 dr, 91% ee, and antineoplastic drugs could be prepared.
[0039] Add nitrogen heterocyclic carbene (3.7 mg, 0.01 mmol) as a catalyst, and 1b (24.3 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol) in sequence in the reaction flask, add 0.5 mL of mesitylene, at 0 °C After 24 hours of reaction, the reaction system was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:5) to obtain the target product 3b (20.8 mg), a white solid, with a yield of 51%, >99 / 1 dr, 88% ee.
[0040] The product 3b was analyzed and t...
Embodiment 3
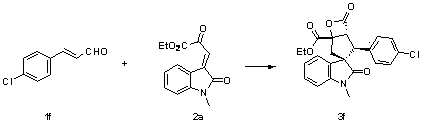
[0042]
[0043] Add nitrogen heterocyclic carbene (3.7 mg, 0.01 mmol) as a catalyst, 1b (26.5 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol), 4Å molecular sieves (10 mg) in sequence, and add 0.5 mL of Toluene was reacted at 0°C for 24 hours. The reaction system was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:10) to obtain the target product 3c (28.3 mg) as a white solid. The rate was 65%, >99 / 1 dr, 86% ee, and enzyme inhibitors could be prepared.
[0044] Add nitrogen heterocyclic carbene (3.7 mg, 0.01 mmol) as a catalyst, and 1b (26.5 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol), 4Å molecular sieves (10 mg) in sequence in the reaction flask, add 0.5 mL tetrahydrofuran, After reacting at 0°C for 24 hours, the reaction system was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:10) to obtain the target product 3c (20.6mg), a white solid, and the yield was 47%, >99 / 1 dr, 81% ee.
[0045] The product 3c was analyzed and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com