Preparation method of chiral alpha-amino acid derivatives
A technology for amino acids and derivatives, applied in the field of preparation of chiral α-amino acid derivatives, which can solve the problem of inability to obtain chiral α-amino acids and their derivatives with high efficiency and high stereoselectivity, low diastereoselectivity, and applicable The scope is limited and other problems, to achieve the effect of avoiding the use of precious metals and highly toxic drugs, high selectivity, and avoiding cumbersome steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0036] Examples 1-9: Add the chiral sulfinyl ester imine compound (0.2 mmol) into the organic reaction solvent, add the halogenated metal reagent at the reaction temperature listed in Table 1, and the reaction time is 0.2~2 hours. After the thin-plate chromatography detects the reaction, add it The ammonium chloride solution was quenched, the aqueous phase was extracted with an organic solvent, the combined organic phases were washed with saturated brine, then dried over anhydrous sodium sulfate, concentrated to dryness under reduced pressure, and subjected to silica gel column chromatography with petroleum ether and ethyl acetate (volume Ratio 2:1~1:1) Purify, and obtain chiral α-amino acid derivatives. The yield and diastereoselectivity are shown in Table 1.
[0037] The structural formula of chiral sulfinyl ester imine compound is:
[0038]
[0039] R 2 Is ethyl, R 3 It is tert-butyl.
[0040] The structural formula of halogenated metal reagent is: R 1 -ZnX·MgX' 2 ·LiX''
[0041...
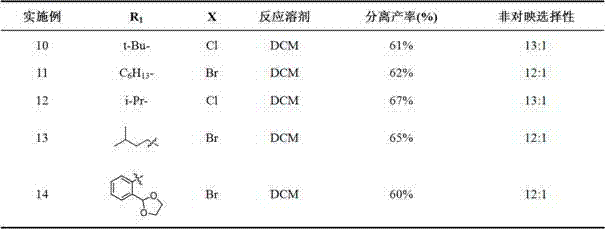
Embodiment 10-14
[0047] Example 10-14: The chiral sulfinyl ester imine compound (0.2 mmol) was added to DCM, and the metal halide reagent was added at -78°C. The reaction time was 0.2 to 2 hours. After the reaction was detected by thin plate chromatography After adding ammonium chloride solution to quench, the aqueous phase was extracted with organic solvent, the combined organic phase was washed with saturated brine, then dried over anhydrous sodium sulfate, concentrated under reduced pressure to dryness, and subjected to silica gel column chromatography with petroleum ether and ethyl acetate (2:1~1:1) Purification to obtain chiral α-amino acid derivatives 。 The yield and diastereoselectivity are shown in Table 2.
[0048] The structural formula of chiral sulfinyl ester imine compound is:
[0049]
[0050] R 2 Is ethyl, R 3 It is tert-butyl.
[0051] The structural formula of halogenated metal reagent is: R 1 -ZnX·MgX' 2 ·LiX''
[0052] R 1 , X is shown in Table 2, or X’ and X’’ are both chlorine. ...
Embodiment 10
[0057] Example 10: 1 H NMR (300 MHz, CDCl 3 ) δ 4.23 (q, J = 7.1, 2H), 3.52 (d, J = 9.5, 1H), 2.06 (d, J = 3.7, 1H), 1.30 (t, J = 5.4, 3H), 1.27 (s, 9H), 0.97 (s, 9H).
[0058] Its structural formula is:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com