Preparation method of organic boron-nitrogen fluorescent compound
A fluorescent compound and boron-nitrogen technology, which is applied in the field of preparation of organic boron-nitrogen fluorescent compounds, can solve the problems of inconvenient industrialization, long reaction time and high reaction temperature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
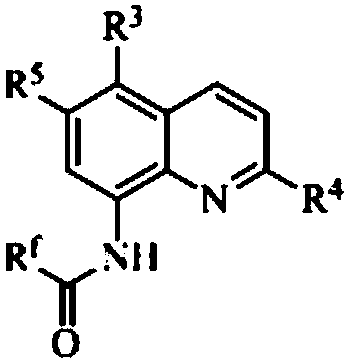
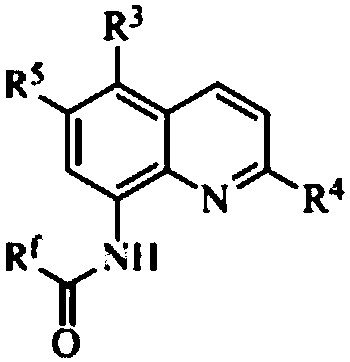
[0030] Add 0.1 mmol I (wherein R f = CF 3 ; 3 , R 4 , R 5 =H), 0.1mmol II (wherein R 1 , R 2 , R 6 =H), 0.005mmol copper bromide, 0.1mmol R 7 COOH (R 7 =Ph is benzene) and 1mL toluene solution, under nitrogen atmosphere, the reaction is carried out at 90 ° C. 5. After the reaction is completed, filter, concentrate, and obtain III (wherein R f= CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 = H, R 7 =Ph), the yield was 82%. Detection of catalytic activity: 0.5mmol aniline (Ar=Ph), 0.5mmol benzaldehyde (Ar=Ph), 0.6mmol 4-phenylcyclohexanone, 0.002 mmol III (where R f = CF 3 ;R 1 , R 2 , R 3 , R 4 , R 5 , R 6 = H, R 7 =Ph) and 1mL toluene (toluene solution), the reaction was carried out at 80°C for 12h, and TLC tracked the reaction until the reaction was complete. The reaction result was: (E)-2-benzyl-4-phenylcyclohexanone, and the yield was 95%, the selectivity of (E)-2-benzyl-4-phenylcyclohexanone is 100%, and the cis-trans selectivity is 1 / 99.
preparation example 2
[0032] Add 0.1 mmol I (wherein R f = CF 3 ; 3 , R 4 , R 5 =H), 0.1mmol II (wherein R 1 , R 2 , R 6 =H), 0.005mmol sodium bromide, 0.1mmol R 7 COOH (R 7 =Ph) and 1mL touluene, under a nitrogen atmosphere, the reaction was carried out at 70°C for 3h. After the reaction was completed, filtered, concentrated, and separated by chromatography to obtain III (wherein R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 = H, R 7 =Ph), the yield was 83%. Detection of catalytic activity: add 0.5mmol aniline (Ar=Ph), 0.5mmol benzaldehyde (Ar=Ph), 0.6mmol 4-methoxycyclohexanone, 0.002mmol III (where R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 = H, R 7 =Ph) and 1mL toluene, the reaction was carried out at 80°C for 12h, TLC followed the reaction until the reaction was complete. The reaction result was: (E)-2-benzyl-4-methoxycyclohexanone, the yield was 97%, The selectivity of (E)-2-benzyl-4-methoxycyclohexanone was 100%, and the cis-trans selectivity was 1 / 99.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com