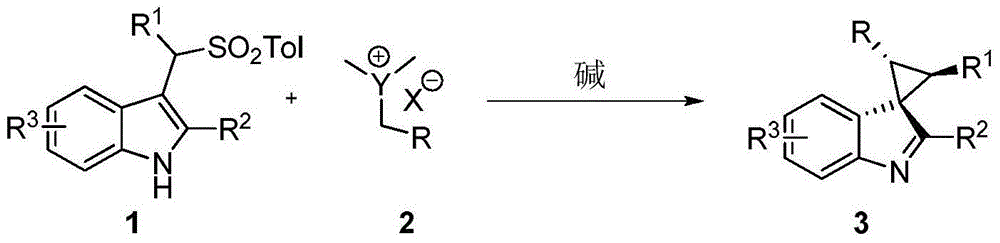
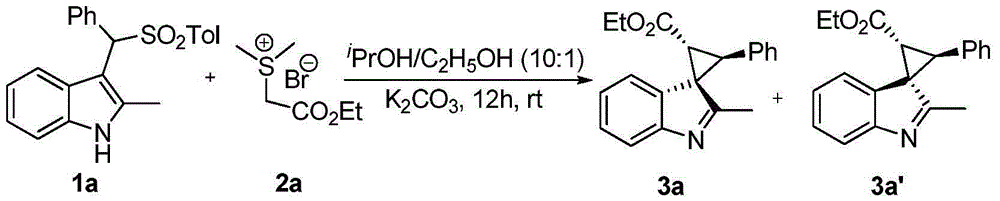Method for synthesizing substituted cyclopropane compounds through dearomatizing indole
A compound and aromatization technology, applied in organic chemistry and other directions, can solve the problems of poor chemical selectivity and stereoselectivity of products, expensive reagents or catalysts, etc., and achieve the effects of complete conversion of raw materials, convenient separation and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: condition optimization
[0030]
[0031] In a nitrogen-protected reaction flask, add substituted indole 1a (0.25 mmol), sulfur ylide 2a (0.38 mmol), potassium carbonate (0.75 mmol), and then add 3 ml of dry isopropanol and 0.3 ml of ethanol. After reacting at room temperature for 12 h, 3 mL of water was added to quench the reaction. After standing still for liquid separation, the aqueous layer was extracted three times with dichloromethane (3×15 mL). After combining the organic layers, they were dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, and the product was obtained by silica gel column chromatography, and the reaction structure was as follows:
[0032]
[0033] Using the same conditions as above, except that different solvents and bases were used, the yield of the product is shown in Table 1.
[0034] Table 1. Optimization of conditions for the synthesis of cyclopropane compounds by dearomatization of indo...
Embodiment 2
[0036] Example 2: Dearomatization of substituted indoles to synthesize cyclopropane compound 3
[0037] In a nitrogen-protected reaction flask, add substituted indole 1a (0.25 mmol), sulfur ylide 2a (0.38 mmol), potassium carbonate (0.75 mmol), and then add 3 ml of dry isopropanol and 0.3 ml of ethanol. After reacting at room temperature for 12 h, 3 mL of water was added to quench the reaction. After standing still for liquid separation, the aqueous layer was extracted three times with dichloromethane (3×15 mL). After combining the organic layers, they were dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, and the product was obtained by silica gel column chromatography, and the reaction structure was as follows:
[0038]
[0039] Using the same conditions as above, except that different compounds 1 and 2 were used, the yields of the products are shown in Table 2.
[0040] Table 2. Dearomatization of substituted indole to cyclopropane co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com