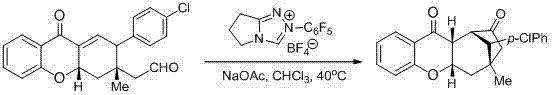Compound containing chiral chromanone skeletons and asymmetric synthetic method thereof
A technology for dihydrochromone and chiral compounds, which is applied in the field of chiral dihydrochromone skeleton compounds and asymmetric synthesis, achieving the effects of mild reaction conditions, simple operation and wide application range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
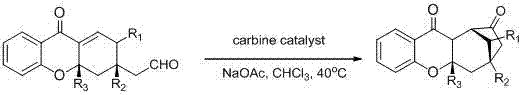
[0021] Example 1: asymmetric anti-electron demand Diels-Alder reaction and series reaction of chromone electron-deficient diene compound and alkenal catalyzed by a, a-diarylprolinol silyl ether.
[0022] In a clean reaction tube, add chiral prolinol silyl ether catalyst (0.02 mmol), chromone electron-deficient diene compound (0.1 mmol), enal (0.2 mmol), acidic additive (0.02 mmol) and 1,4-Dioxane (1 mL) at 25 o The reaction time was stirred under C, and after the reaction was monitored by TLC, the solvent was recovered under reduced pressure, and the residue was separated by column chromatography to obtain the target product.
[0023] P1, 89% yield, 94% ee , Chiral test conditions: HPLC analysis on Chiralpak IC column (20% 2-propanol / n -hexane, 1 mL / min), UV 220 nm, t major = 23.47 min, t minor = 25.67 min. [α] D 20 = -21.3 ( c = 0.79 in CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ): 8.01 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H), 7.52 (td, J = 8.0 Hz, J = 1.6 Hz, ...
Embodiment 2
[0047] Example 2: Transformation with multifunctional functional groups (application example 1)
[0048]
[0049] P22 (37 mg, 0.1 mmol) was weighed into a dry reaction tube, dissolved in 1 mL of chloroform, and then the carbene catalyst (7.3 mg, 0.02 mmol) and sodium acetate (9.8 mg, 0.12 mmol), heated to 40°C and stirred until P22 disappeared as detected by thin layer chromatography. Column chromatography separated petroleum ether / ethyl acetate: 8 / 1) to obtain the title compound, 92% yield, 90% ee , Chiral test conditions: HPLC analysis on Chiralpak OD column (20% 2-propanol / n -hexane, 1 mL / min), UV 220 nm, t minor = 11.96 min, t major = 17.04 min. [α] D 20 = +128.8 ( c = 0.90 in CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ): 7.92 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H), 7.50 (td, J = 8.0 Hz, J = 1.6 Hz, 1H), 7.27-7.25 (m, 2H), 7.07 (t, J = 8.0 Hz, 1H), 6.95-6.92 (m, 3H), 4.79-4.78 (m, 1H), 3.10 (t, J = 3.6 Hz, 1H), 2.99 (br s, 2H), 2.71 (d, J = 18.4 Hz, 1H),...
Embodiment 3
[0050] Example 3: Transformation with multifunctional functional groups (application example 2)
[0051]
[0052] P22 (33 mg, 0.09 mmol) was weighed into a dry reaction tube, dissolved in 1 mL of dichloromethane, and organic base DBU (15.2 mg, 0.1 mmol), stirred at room temperature until P22 disappeared as detected by thin layer chromatography. Column chromatography separated petroleum ether / ethyl acetate: 10 / 1) to obtain the title compound as a semi-solid, 88% yield, 94% ee , Chiral test conditions: HPLC analysis on Chiralpak AD column (20% 2-propanol / n -hexane, 1 mL / min), UV 254 nm, t major = 11.14 min, t minor = 19.03 min. [α] D 20 = -17.0 ( c = 0.50 in CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ): 8.03 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H), 7.52 (td, J = 8.4 Hz, J = 1.6 Hz, 1H), 7.31 (d, J = 8.4 Hz, 2H), 7.12-7.07 (m, 3H), 6.99 (d, J = 8.4 Hz, 1H), 6.33 (s, 1H), 4.60 (t, J = 6.0 Hz, 1H), 4.48 (dd, J = 10.0 Hz, J = 2.8 Hz, 1H), 2.30 (dd, J = 13.6 Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com