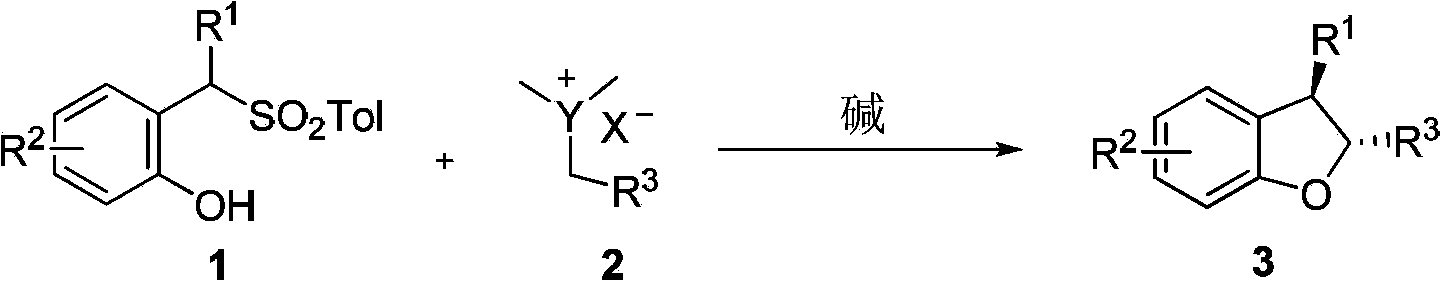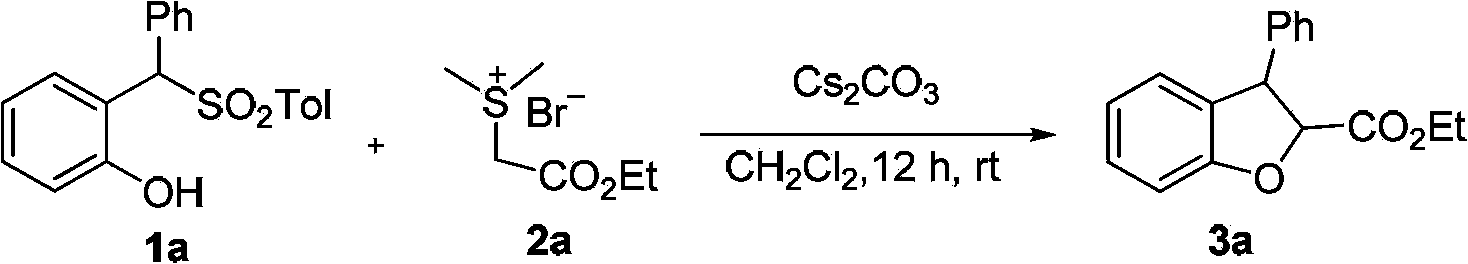Method for synthetizing 2,3-disubstituted dihydrobenzofuran
A benzodihydrofuran and disubstituted technology, applied in the field containing dihydrobenzofuran, can solve the problems of poor chemical selectivity and stereoselectivity of products, harsh reaction conditions, expensive reaction reagents or catalysts, etc., and achieve complete conversion of raw materials. , the effect of easy availability of raw materials and convenient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Synthesis of compound 3a
[0031]
[0032] Add substituted phenol 1a (1.0 mmol), sulfur ylide 2a (1.2 mmol), and cesium carbonate (2.5 mmol) into a reaction flask protected by nitrogen, and then add 4 ml of dry dichloromethane. After 12 hours of reaction at room temperature, saturated sodium thiosulfate solution was added to quench the reaction. After standing for liquid separation, the aqueous layer was extracted three times with dichloromethane (3×5 mL), and the organic layers were combined and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure and silica gel column chromatography was used to obtain the product compound 3a.
[0033] tran-Ethyl 3-phenyl-2,3-dihydrobenzofuran-2-carboxylate(3a). White solid, melting point 59-60°C, yield 99%, 1 H NMR(400MHz, CDCl 3 )δ7.35-7.20(m,6H), 7.00(dd,J=7.4,4.1Hz,2H), 6.91(t,J=7.4Hz,1H), 5.03(d,J=6.6Hz,1H), 4.81(d,J=6.5Hz,1H), 4.29(ddd,J=12.5,7.5,2.8Hz,2H), 1.31(t,J=7.1Hz,3H); 13 C NMR(100MH...
Embodiment 2
[0034] Example 2: Synthesis of compound 3b
[0035]
[0036] Add substituted phenol 1b (1.0 mmol), sulfur ylide 2a (1.2 mmol), and cesium carbonate (2.5 mmol) into a reaction flask protected by nitrogen, and then add 4 ml of dry dichloromethane. After 12 hours of reaction at room temperature, saturated sodium thiosulfate solution was added to quench the reaction. After standing for liquid separation, the aqueous layer was extracted three times with dichloromethane (3×5 mL), and the organic layers were combined and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure and silica gel column chromatography was used to obtain the product compound 3b.
[0037] tran-Ethyl 3-ethyl-2,3-dihydrobenzofuran-2-carboxylate(3j). Colorless liquid, yield 77%; 1 H NMR(400MHz, CDCl 3 )δ7.16(t,J=7.8Hz,2H),6.91-6.88(m,2H),4.82(d,J=5.4Hz,1H),4.33-4.18(m,2H),3.50(dd,J =12.7,5.7Hz,1H),1.81(ddd,J=21.4,10.7,6.2Hz,2H),1.29(t,J=7.1Hz,3H),1.03(t,J=7.4Hz,3H); 13 C NMR(100MHz, CDC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com