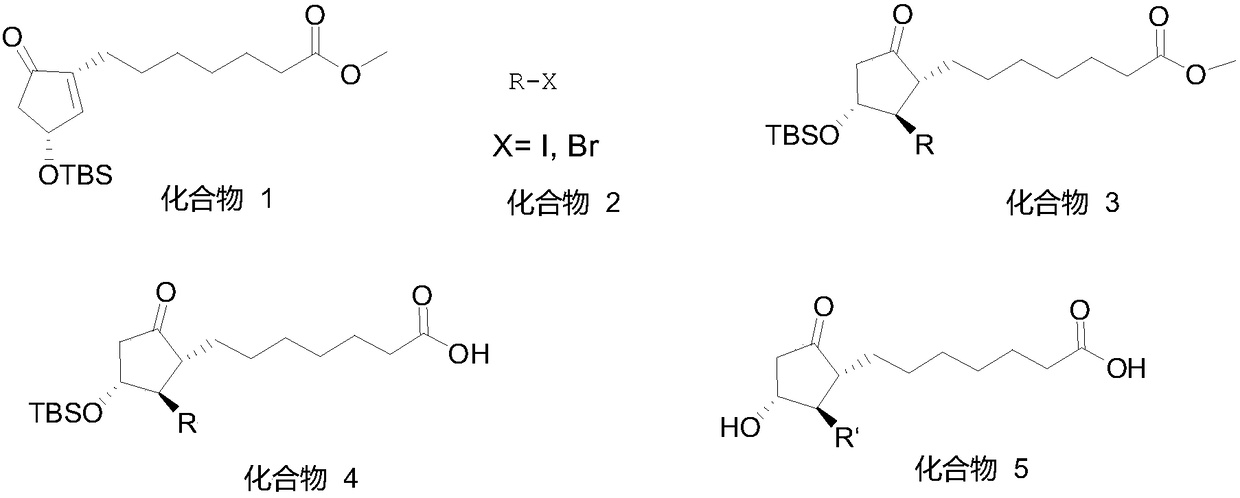
Method for synthesizing 1R,2R,3R-substituted cyclopentanone compound
A synthesis method and compound technology, which is applied in the field of synthesis of 1R, 2R, 3R-substituted cyclopentanone compounds, can solve the problems of low diastereoselectivity, difficulty in industrialization, and low purity of products, and achieve diastereometry. High selectivity, easy reaction control, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The synthesis of embodiment 1 prostaglandin E1 (compound 5b)
[0042]
[0043] (1) Synthesis of compound 3b
[0044]
[0045] Under the protection of argon, cuprous iodide (0.50g, 5.65mmol) was added to a dry round bottom flask, tetrahydrofuran (3ml) was added, it was cooled to -78°C, tert-butyllithium was added dropwise Alkane solution (4.2ml, 6.78mmol), stirred for 2h, and slowly heated to 40°C for 30 minutes to obtain the first reaction solution, which was set aside.
[0046] Under the protection of argon at -78°C, add n-butyl lithium in n-hexane (4.2ml, 6.78mmol) dropwise to a solution of compound 2b (2.08g, 5.65mmol) in tetrahydrofuran (3ml), and stir at -78°C 2 hours; then slowly add the first reaction solution dropwise into the reaction system, react at -78°C for 2 hours, then raise the temperature to 40°C and react for 30 minutes, then cool down to -78°C to obtain the second reaction solution, ready for use .
[0047]Under the protection of argon at -78...
Embodiment 2
[0054] Synthesis of Example 2 Limatoprost Analog (Compound 5c)
[0055]
[0056] (1) Synthesis of compound 3c
[0057]
[0058] Replace 2b in Example 1 with 2c, and its synthesis method is the same as step (1) in Example 1. Yield, 82%. 1 HNMR (CDCl 3 ,400MHz):5.68(dd,J=15.6and 5.2Hz,1H),5.55(dd,J=15.6,7.6Hz,1H),4.15-4.05(m,2H),3.68(s,3H),2.65( dd, J=18.4and 6.8Hz, 1H), 2.46(m, 1H), 2.28(t, J=7.6Hz, 2H), 2.21(dd, J=18.4and 8.0Hz, 1H), 1.93(m, 2H ), 1.63–1.26(m,17H), 0.91-0.89(m,24H), 0.01(s,12H). MS (m / z): 642 (M+1). de>96%.
[0059] (2) Synthesis of compound 4c
[0060]
[0061] Use 3c to replace 3b in Example 1, and its synthesis method is the same as step (2) in Example 1. Yield, 95%. 1 HNMR (CDCl 3 ,400MHz):5.67(dd,J=15.6and 5.2Hz,1H),5.54(dd,J=15.6,7.6Hz,1H),4.15-4.05(m,2H),2.63(dd,J=18.4and 6.8 Hz, 1H), 2.45(m, 1H), 2.27(t, J=7.6Hz, 2H), 2.20(dd, J=18.4and 8.0Hz, 1H), 1.92(m, 2H), 1.62–1.26(m ,17H),0.91-0.89(m,24H),0.01(s,12H). MS (m / z): 628 (M+1). ...
Embodiment 3
[0065] Synthesis of embodiment 3 misoprostol analog (compound 5d)
[0066]
[0067] (1) Synthesis of compound 3d
[0068]
[0069] Replace 2b in Example 1 with 2d, and its synthesis method is the same as step (1) in Example 1. Yield, 82%. 1 HNMR (CDCl 3 ,400MHz):5.58(dd,J=15.0and 5.2Hz,1H),5.49(dd,J=15.0,7.2Hz,1H),4.11-4.00(m,2H),3.65(s,3H),2.60( dd, J=17.6and 6.8Hz, 1H), 2.43(m, 1H), 2.24(t, J=7.6Hz, 2H), 2.18(dd, J=17.6and 8.0Hz, 1H), 1.91(m, 2H ), 1.59–1.23(m,16H), 0.88-0.90(m,24H), 0.01(s,12H). MS (m / z): 628 (M+1). de>96%.
[0070] (2) Synthesis of compound 4d
[0071]
[0072] Replace 3b in Example 1 with 3d, and its synthesis method is the same as step (2) in Example 1. Yield, 96%. 1 HNMR (CDCl 3 ,400MHz):5.57(dd,J=15.0and 5.2Hz,1H),5.48(dd,J=15.0,7.2Hz,1H),4.10-4.00(m,2H),2.60(dd,J=17.6and 6.8 Hz, 1H), 2.42(m, 1H), 2.23(t, J=7.6Hz, 2H), 2.17(dd, J=17.6and 8.0Hz, 1H), 1.90(m, 2H), 1.57–1.23(m ,16H),0.87-0.90(m,24H),0.01(s,12H). MS (m / z): 628 (M+1). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com