Synthesis method of polysubstituted 4-phenyl chroman compounds
A synthesis method, phenyl color technology, applied in the direction of organic chemistry, can solve the problems of using noble metal catalysts, harsh synthesis conditions, etc., to achieve excellent diastereoselectivity, convenient post-processing, yield and diastereoselectivity maintenance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The reaction equation is as follows:
[0026]
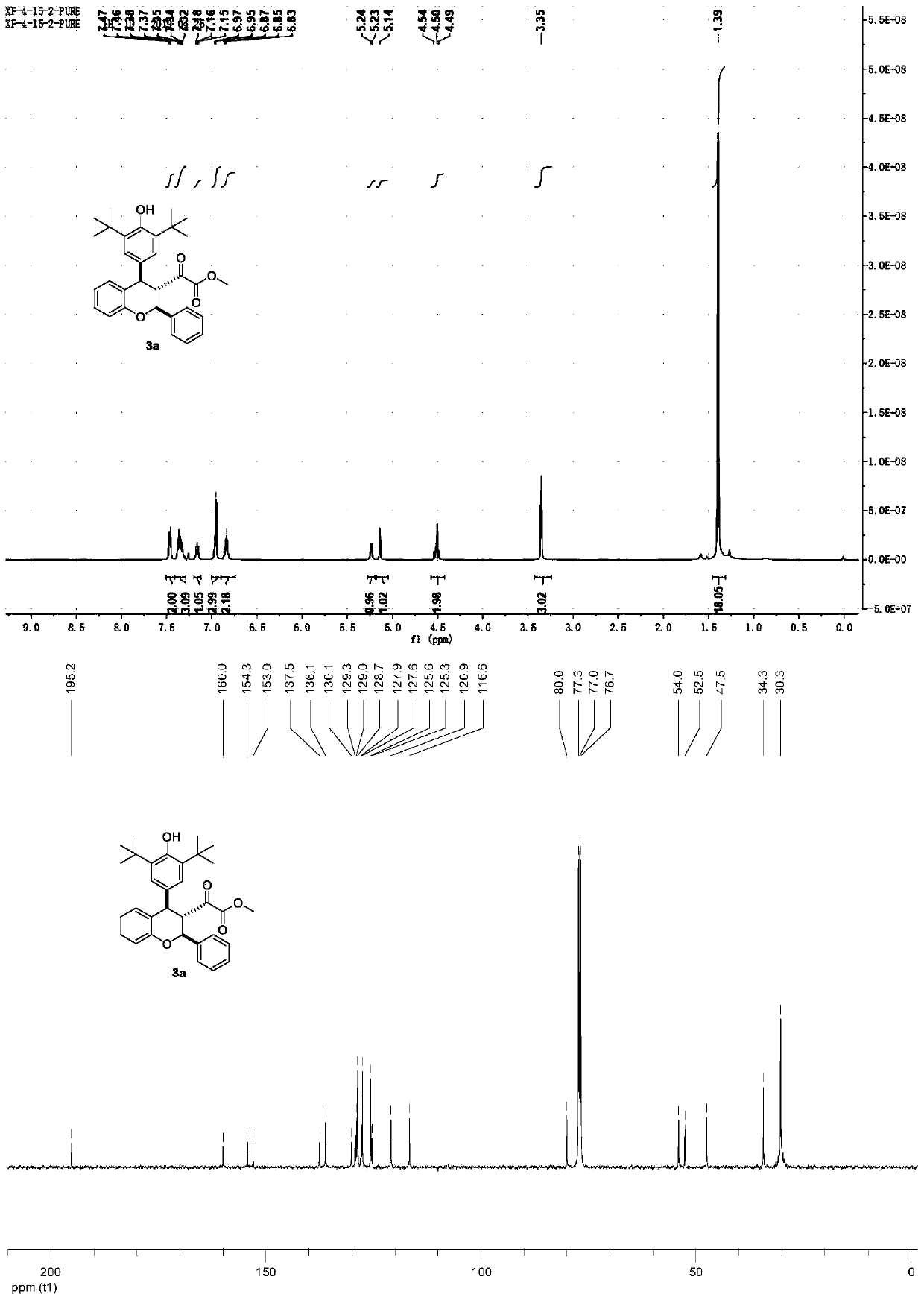
[0027] Add compound 1a (100mmol) and compound 2a (150mmol) into the reactor, add 1000mL 1,2-dichloroethane solution, then add 1,8-diazabicyclo[5.4.0]undec-7- Alkene (150mmol), while adding Molecular sieves (50m%), stirred at room temperature for 40 hours. After the reaction, the reaction system was distilled off under reduced pressure to remove 1,2-dichloroethane to obtain a crude product. The crude product was added to a silica gel chromatography column, using a mixed solvent of petroleum ether / ethyl acetate (V / V=30 / 1) as the eluent, and column chromatography gave 3a as a white solid. The yield of 3a was 85%, and the diastereoselectivity was greater than 20:1.
[0028] The NMR data of 3a are as follows:
[0029] 1 H NMR (500MHz, CDCl 3 )δ(ppm): 7.46(d, J=7.1Hz, 2H), 7.32-7.38(m, 3H), 7.16(t, J=7.2Hz, 1H), 6.96(d, J=7.8Hz, 3H) ,6.82(m,2H),5.23(d,J=6.9Hz,1H),5.14(s,1H),4.49-4.54(m,2H),3.35(s,3H),1.39(s,18H).
[...
Embodiment 2
[0032] The reaction equation is as follows:
[0033]
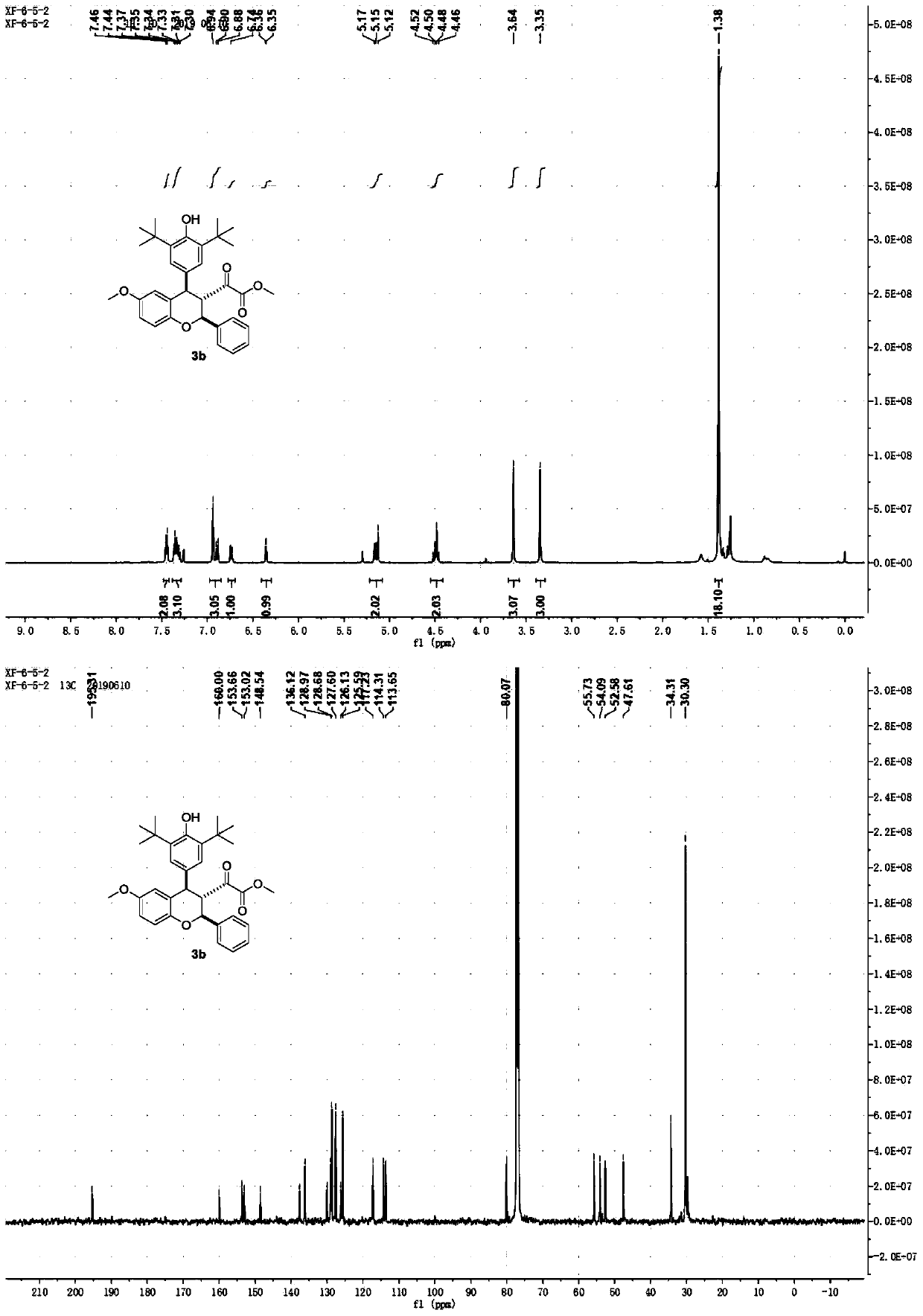
[0034] Add compound 1b (0.1mmol) and compound 2a (0.15mmol) into the reactor, add 1mL 1,2-dichloroethane solution, and then add 1,8-diazabicyclo[5.4.0]undeca- 7-ene (0.15mmol), while adding Molecular sieves (50m%), stirred at room temperature for 40 hours. After the reaction, the reaction system was distilled off under reduced pressure to remove 1,2-dichloroethane to obtain a crude product. The crude product was added to a silica gel chromatography column, using a mixed solvent of petroleum ether / ethyl acetate (V / V=30 / 1) as the eluent, and column chromatography gave 3b as a white solid. The yield of 3b was 80%, and the diastereoselectivity was greater than 20:1.
[0035] The NMR data of 3b are as follows:
[0036] 1 H NMR (500MHz, CDCl 3 )δ (ppm): 7.45 (d, J = 7.2Hz, 2H), 7.30-7.37 (m, 3H), 6.94 (s, 2H), 6.89 (d, J = 8.8Hz, 1H), 6.74 (dd, J=8.8,2.5Hz,1H),6.36(d,J=2.5Hz,1H),5.16(d,J=8.4Hz,1H),5.12(s,1H),4.46-4.52...
Embodiment 3
[0039] The reaction equation is as follows:
[0040]
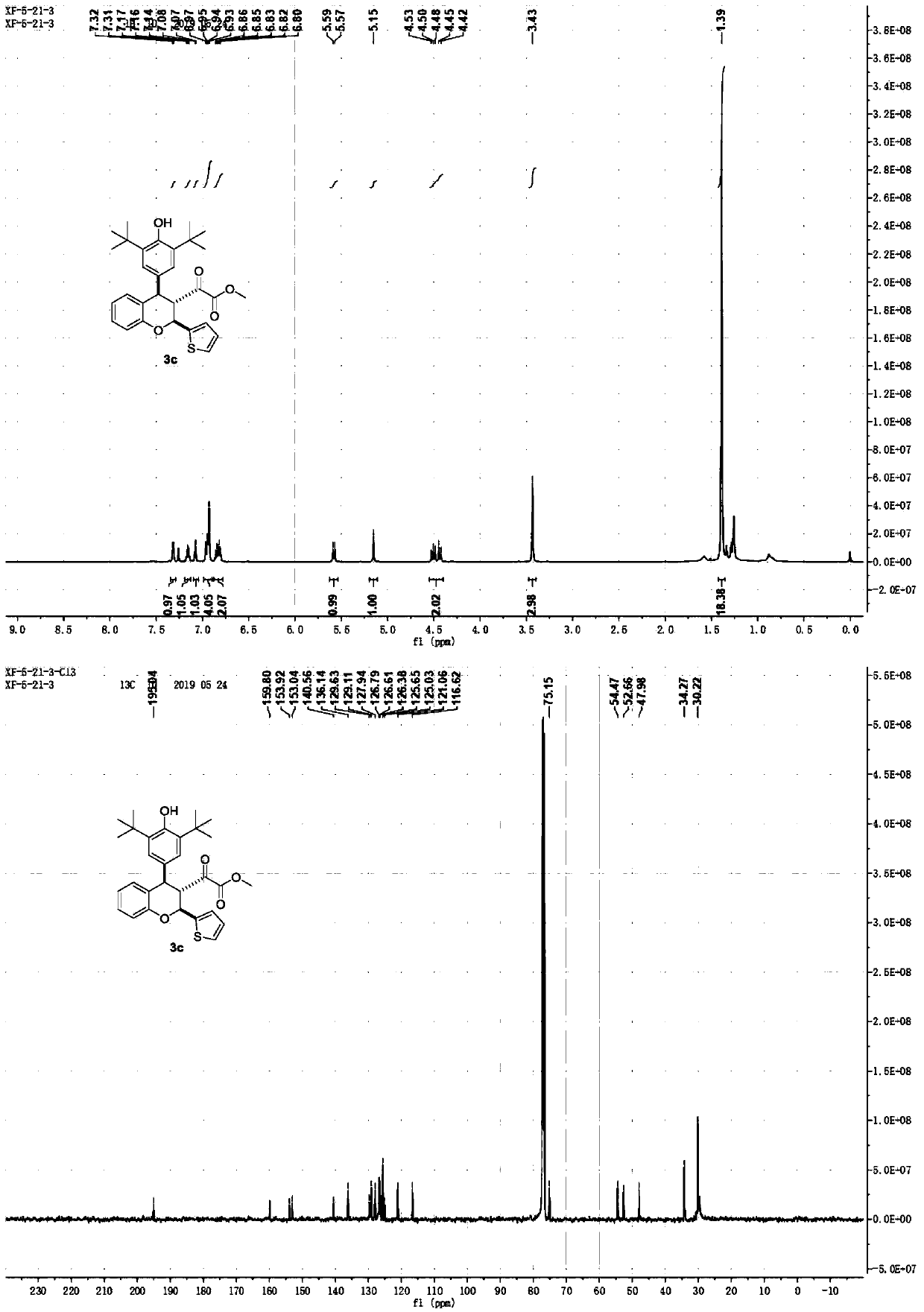
[0041] Add compound 1a (0.1mmol) and compound 2b (0.15mmol) into the reactor, add 1mL 1,2-dichloroethane solution, then add 1,8-diazabicyclo[5.4.0]undeca- 7-ene (0.15mmol), while adding Molecular sieves (50m%), stirred at room temperature for 40 hours. After the reaction, the reaction system was distilled off under reduced pressure to remove 1,2-dichloroethane to obtain a crude product. The crude product was added to a silica gel chromatography column, using a mixed solvent of petroleum ether / ethyl acetate (V / V=30 / 1) as the eluent, and column chromatography gave 3c as a white solid. The yield of 3c was 56% with a diastereoselectivity greater than 20:1.
[0042] The NMR data of 3c are as follows:
[0043] 1 H NMR (500MHz, CDCl 3 )δ(ppm): 7.32(d, J=4.6Hz, 1H), 7.16(t, J=7.3Hz, 1H), 7.08(d, J=2.2Hz, 1H), 6.93-6.97(m, 4H) ,6.80-6.86(m,2H),5.58(d,J=9.9Hz,1H),5.15(s,1H),4.42-4.53(m,2H),3.43(s,3H),1.39(s,18H ).
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com