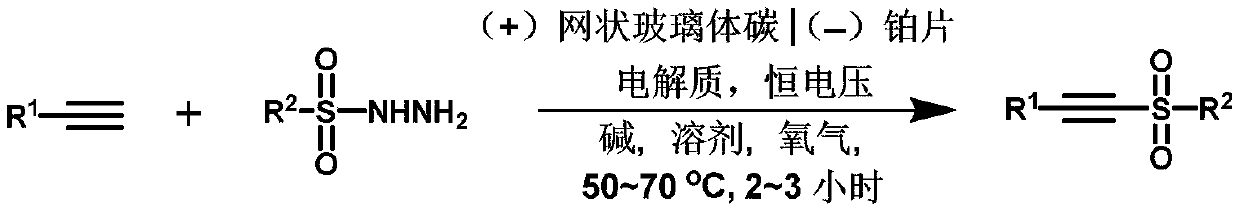
Method for synthesizing alkyne sulfone compounds from terminal alkyne and sulfonyl hydrazine
A terminal alkyne, sulfonyl hydrazide technology, applied in the field of chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
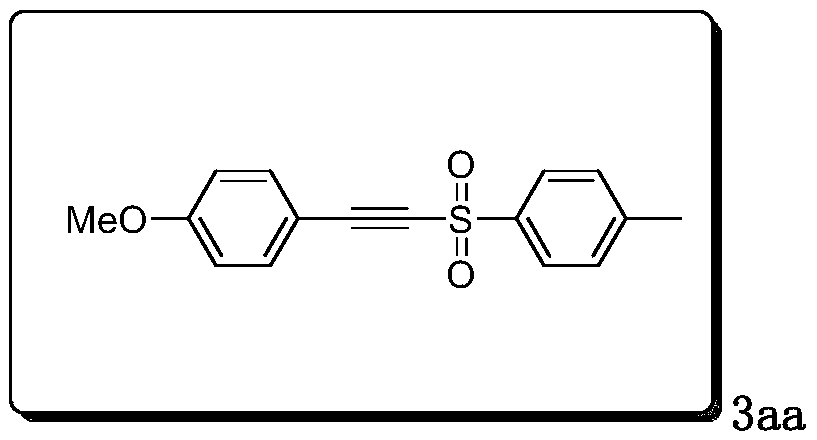
[0021] Preparation and characterization of 1-methoxy-4-(tosylethynyl)benzene (3aa):
[0022]
[0023] Add 0.25mmol of p-methoxyphenylacetylene, 1mmol of p-toluenesulfonyl hydrazide, 2 equivalents of potassium iodide and 2 equivalents of cesium carbonate to a 25mL three-neck flask, add 18mL of acetonitrile and water mixed solution to dissolve, and use reticulated glassy carbon RVC was used as the anode and the platinum sheet was used as the cathode. The stirring reaction was carried out at 1.0V constant voltage, oxygen atmosphere, and 50°C for 2 hours. TLC monitored the reaction process. After the reaction was completed, the mixture was extracted with 10 mL ethyl acetate, and the organic layer was used Anhydrous MgSO 4 Drying, the solvent was spin-dried under reduced pressure, and passed through column chromatography (SiO 2 , ethyl acetate / petroleum ether=1:12) to purify the residue to obtain 3aa as pale yellow solid.
[0024] The product is characterized by:
[0025] Pal...
Embodiment 2
[0027] Preparation and characterization of 1-methoxy-4-((benzenesulfonyl)ethynyl)benzene (3ab):
[0028]
[0029] Add 0.25mmol of p-methoxyphenylacetylene, 1mmol of benzenesulfonyl hydrazide, 2 equivalents of ammonium iodide and 2 equivalents of potassium carbonate to a 25mL three-necked flask, add 18mL of acetonitrile and water mixed solution to dissolve, and use a reticulated glass Carbon RVC was used as the anode, and the platinum sheet was used as the cathode. Stirring reaction was carried out at 1.2V constant voltage, oxygen atmosphere, and 40°C for 3 hours. TLC monitored the reaction progress. After the reaction was completed, the mixture was extracted with 10mL ethyl acetate, and the organic layer was with anhydrous MgSO 4 Drying, the solvent was spin-dried under reduced pressure, and passed through column chromatography (SiO 2 , ethyl acetate / petroleum ether=1:10) to obtain 3ab as a yellow oily liquid.
[0030] The product is characterized by:
[0031] Yellow oil...
Embodiment 3
[0033] Preparation and characterization of 1-(((4-methoxyphenyl)ethynyl)sulfonyl)-2-methylbenzene (3ac):
[0034]
[0035] Add 0.25mmol of p-methoxyphenylacetylene, 1mmol of o-toluenesulfonyl hydrazide, 2 equivalents of tetrabutylammonium iodide and 2 equivalents of potassium tert-butoxide to a 25mL three-necked flask, add 18mL of acetonitrile and water The mixed solution was dissolved, and the reticulated vitreous carbon RVC was used as the anode, and the platinum sheet was used as the cathode, and the stirring reaction was carried out under the conditions of 1.4V constant voltage, oxygen atmosphere, and 60°C for 3 hours, and the reaction progress was monitored by TLC. The mixture was extracted with ester, and the organic layer was washed with anhydrous MgSO 4 Drying, the solvent was spin-dried under reduced pressure, and passed through column chromatography (SiO 2 , ethyl acetate / petroleum ether=1:15) to obtain 3ac as a white solid.
[0036] The product is characterized...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com