Biaryl compound as well as preparation method and application thereof
A compound, biaryl technology, applied in the field of biaryl compounds and their preparation, to achieve the effects of high economic benefits, simple post-treatment, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048](1) Weigh magnesium chips (72.9mg, 3mmol, 3equiv.) and lithium chloride (84.8mg, 2mmol, 2equiv.) and add to the schlenk seal tube. Then under reduced pressure, the mixture of magnesium chips and lithium chloride was heated with an electric heat gun (320° C., 3 minutes).
[0049] (2) After the mixture was cooled to room temperature, 3 mL of ultra-dry tetrahydrofuran was added thereto, and then the tube was sealed and replaced with nitrogen three times. Subsequently, phenyl trimethyl quaternary ammonium iodide (263.1 mg, 1 mmol, 1 equiv.), bistriphenylphosphine palladium dichloride (35.1 mg, 0.05 mmol, 5 mol%), 4-methyl Oxybromobenzene (374.1mg, 2mmol, 2equiv.) and N,N,N',N'-tetramethylethylenediamine (232.4mg, 2mmol, 2equiv.), the mixture was stirred at room temperature for 12 hours.
[0050] (3) Subsequent quenching with saturated ammonium chloride solution and extraction with ethyl acetate. After the extract was washed with saturated brine and dried over anhydrous sod...
Embodiment 2~32
[0054] Embodiments 2 to 32 are basically the same as the above-mentioned embodiment 1, except that in step (1), the aryl quaternary ammonium salt and the aryl bromide are different, as shown in Table 1 below:
[0055] Table 1 Examples 2-32
[0056]
[0057]
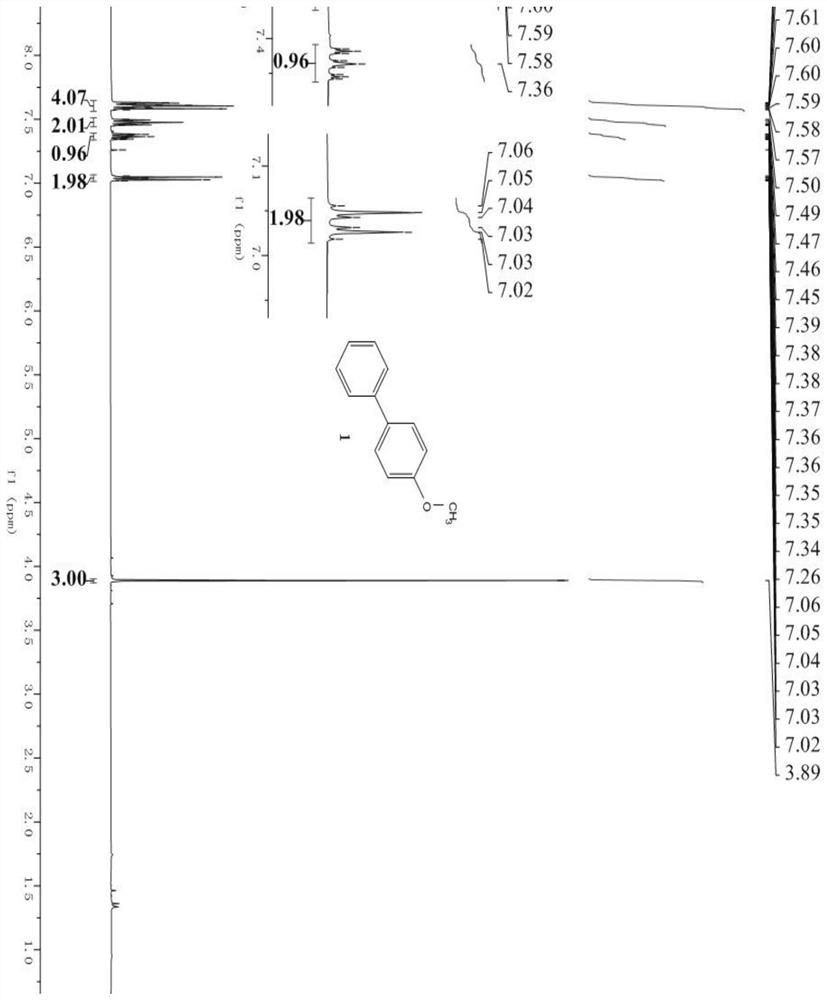
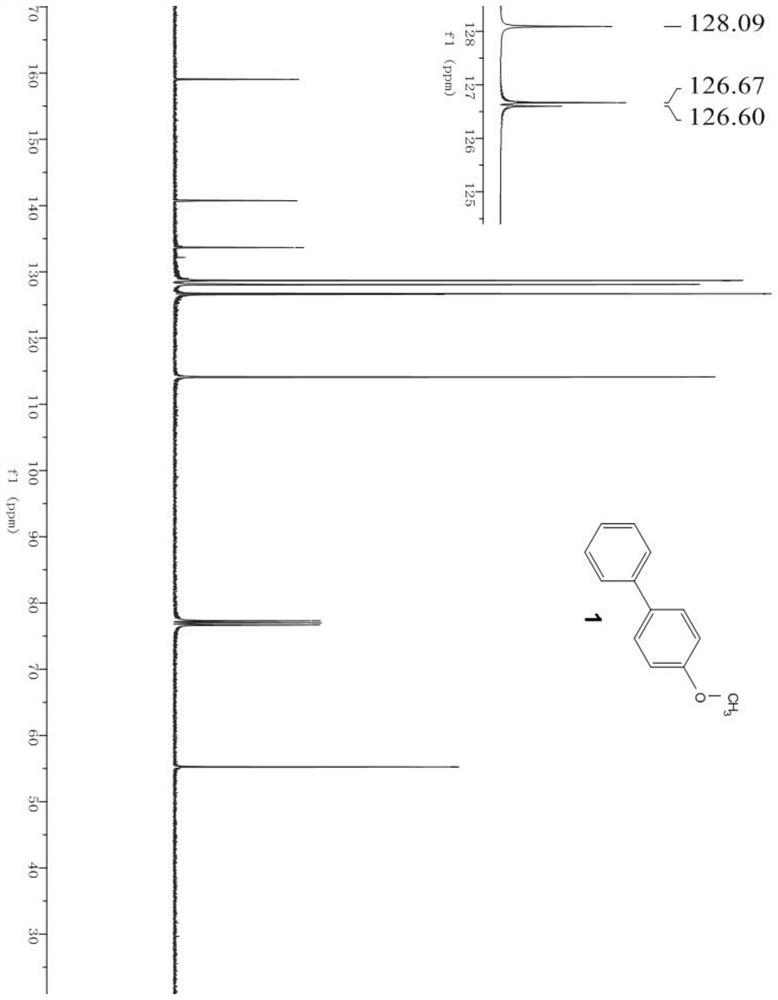
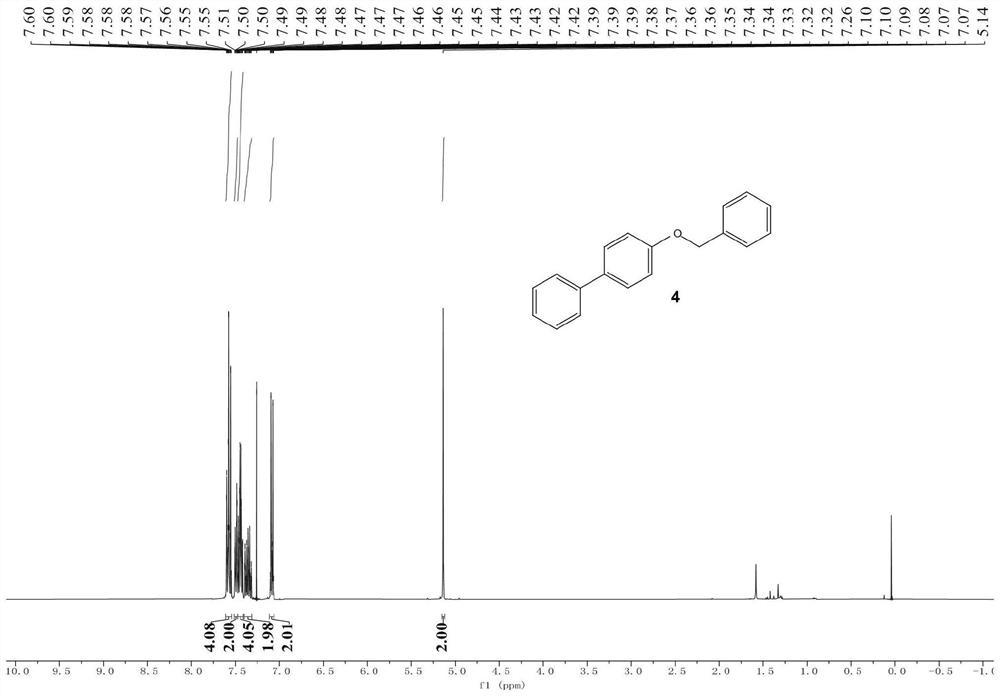
[0058] The products in the above table are randomly selected for characterization, wherein, image 3 is the hydrogen spectrum of product 4 (4-benzyloxybiphenyl), Figure 4 It is the carbon spectrum of product 4 (4-benzyloxybiphenyl); Figure 5 is the hydrogen spectrum of product 6 (3-methoxybiphenyl), Image 6 It is the carbon spectrum of product 6 (3-methoxybiphenyl); Figure 7 is the hydrogen spectrum of product 10 (4-dimethylaminobiphenyl), Figure 8 Is the carbon spectrum of product 10 (4-dimethylaminobiphenyl); Figure 9 is the hydrogen spectrum of product 26 (4-fluoro-4'-methoxy-1,1'-biphenyl), Figure 10 is the fluorine spectrum of product 26 (4-fluoro-4'-methoxy-1,1'-biphenyl), Figure 11 is the carbo...
Embodiment 33~43
[0060] Embodiments 33 to 43 are basically the same as the above-mentioned embodiment 1, except that in step (1), the catalyst, solvent, temperature (°C), and time (h) are different, as shown in Table 2 below:
[0061] Table 2
[0062] Example number catalyst solvent temperature(℃) time (h) Yield(%) 33 Bistriphenylphosphine palladium dichloride N,N-Dimethylformamide 25 12 0 34 Bistriphenylphosphine palladium dichloride DMSO 25 12 0 35 Bistriphenylphosphine palladium dichloride N,N-Dimethylacetamide 25 12 0 36 Bistriphenylphosphine palladium dichloride Acetonitrile 25 12 0 37 Bistriphenylphosphine palladium dichloride 1,4-dioxane 25 12 0 38 Ferric chloride Tetrahydrofuran 25 12 <5
[0063] As can be seen from Table 2, under the same reaction conditions, using bistriphenylphosphine palladium dichloride as a catalyst for the reaction, in N, N-dimethylformamide, DMSO, N, N-dimethylacetamide , aceto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com