A nickel-catalyzed iron-mediated fluoroalkylation of alkynes to synthesize (z)-alkenes and products
A technology for fluoroalkyl and fluoroalkylation of alkynes, applied in the field of organic compound synthesis, can solve the problems of unstable E/Z selectivity, high price, no economical practicability, etc., and expand the scope of preparation of substrates , mild conditions, good applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
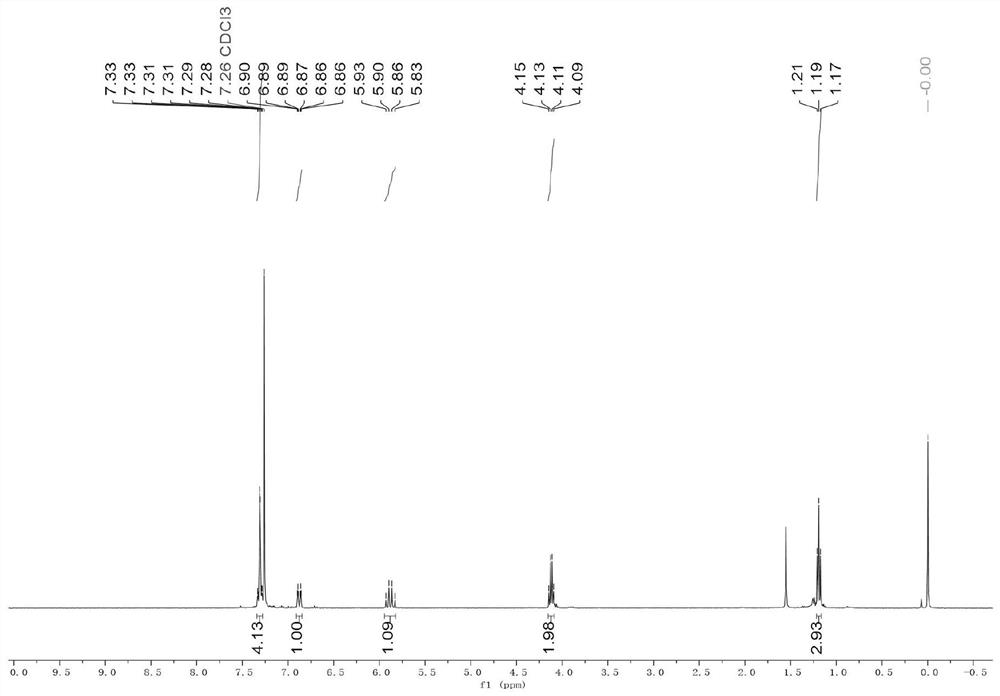
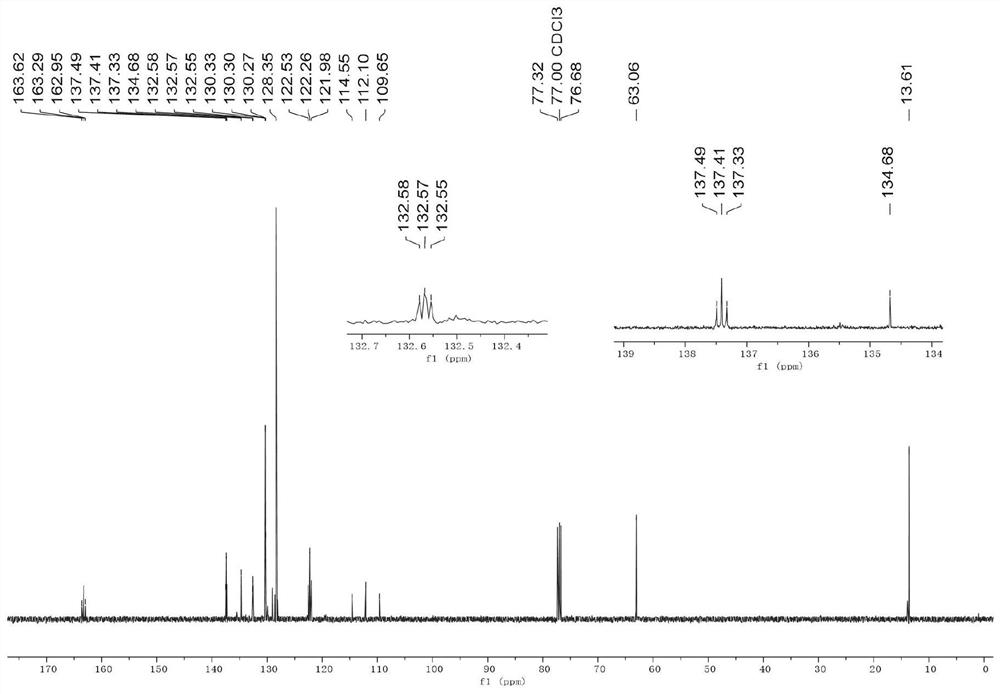
Embodiment 1
[0041] (1) Iron powder (168.0 mg, 3.0 mmol, 3.0 equiv.) and DMF (2 mL) were sequentially added to a 10 mL Schlenk flask. 1,2-Dibromoethane (28mg, 0.15mmol) was added, the reaction flask was heated to 320°C, maintained for 35 seconds and then cooled to room temperature. Then trimethylchlorosilane (22mg, 0.15mmol) was added, heated with an electric heat gun at 320°C for 35 seconds, and then cooled to room temperature to complete the activation of the iron powder.
[0042] (2) After cooling to room temperature, add NiCl to the reaction flask in turn 2(13mg, 0.1mmol, 0.1equiv.), LiI (267.7mg, 2.0mmol, 2.0equiv.), DPEPhos (108mg, 0.2mmol, 0.2equiv.), 2-bromo-2,2-difluoro-3-acetic acid Ethyl ester (3.0 mmol, 3.0 equiv.) and 4-chlorophenylacetylene (1.0 mmol, 1.0 equiv.). The reaction mixture was stirred at 60 °C for 24 h, then washed with saturated NH 4 The Cl solution was quenched and extracted with ethyl acetate (20 mL×3). The combined organic phases were successively washed w...
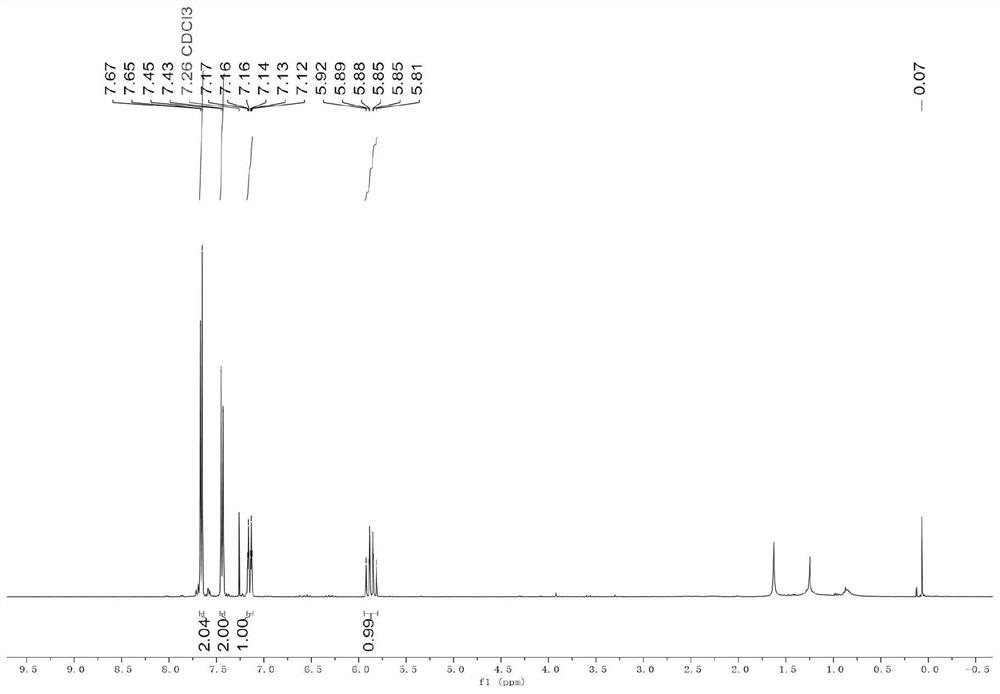
Embodiment 2
[0047] (1) Iron powder (168.0 mg, 3.0 mmol, 3.0 equiv.) and DMF (2 mL) were sequentially added to a 10 mL Schlenk flask. 1,2-Dibromoethane (28mg, 0.15mmol) was added, the reaction flask was heated to 320°C, maintained for 35 seconds and then cooled to room temperature. Then trimethylchlorosilane (22mg, 0.15mmol) was added, heated with an electric heat gun at 320°C for 35 seconds, and then cooled to room temperature to complete the activation of the iron powder.
[0048] (2) After cooling to room temperature, add NiCl to the reaction flask in turn 2 (13mg, 0.1mmol, 0.1equiv.), LiI (267.7mg, 2.0mmol, 2.0equiv.), DPEPhos (108mg, 0.2mmol, 0.2equiv.), perfluoroiodoethane (3.0mmol, 3.0equiv.) and 4-cyanophenylacetylene (1.0 mmol, 1.0 equiv.). The reaction mixture was stirred at 100 °C for 24 h, then washed with saturated NH 4 The Cl solution was quenched and extracted with ethyl acetate (20 mL×3). The combined organic phases were successively washed with saturated brine (20 mL),...
Embodiment 3
[0053] (1) Iron powder (168.0 mg, 3.0 mmol, 3.0 equiv.) and DMF (2 mL) were sequentially added to a 10 mL Schlenk flask. 1,2-Dibromoethane (28mg, 0.15mmol) was added, the reaction flask was heated to 320°C, maintained for 35 seconds and then cooled to room temperature. Then trimethylchlorosilane (22mg, 0.15mmol) was added, heated with an electric heat gun at 320°C for 35 seconds, and then cooled to room temperature to complete the activation of the iron powder.
[0054] (2) After cooling to room temperature, add NiCl to the reaction flask in turn 2 (13mg, 0.1mmol, 0.1equiv.), LiI (267.7mg, 2.0mmol, 2.0equiv.), DPEPhos (108mg, 0.2mmol, 0.2equiv.), 2-bromo-2,2-difluoro-3-acetic acid Ethyl ester (3.0 mmol, 3.0 equiv.) and 2-ethynylbenzyl 2-(4-isobutylphenyl)propionate (1.0 mmol, 1.0 equiv.). The reaction mixture was stirred at 60 °C for 24 h, then washed with saturated NH 4 The Cl solution was quenched and extracted with ethyl acetate (20 mL×3). The combined organic phases we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com