A kind of nickel-catalyzed direct reductive cross-coupling method and product of heterocyclic phosphonium salt and aryl bromide
A cross-coupling, nickel-catalyzed technology, applied in chemical instruments and methods, organic chemistry, organic substitution, etc., to achieve the effect of green steps, mild reaction conditions and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
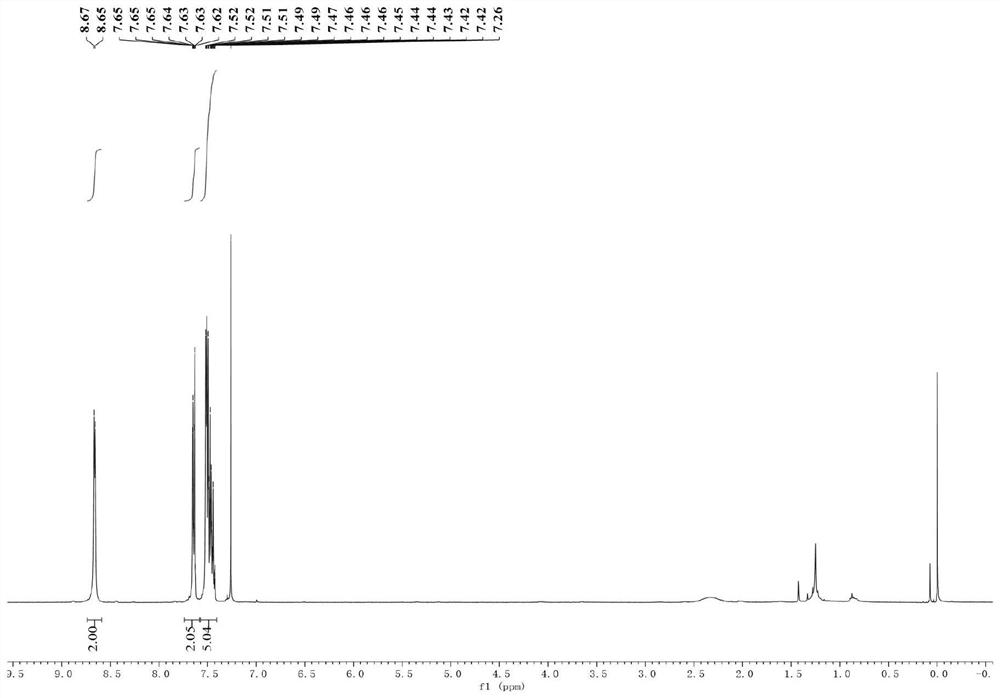
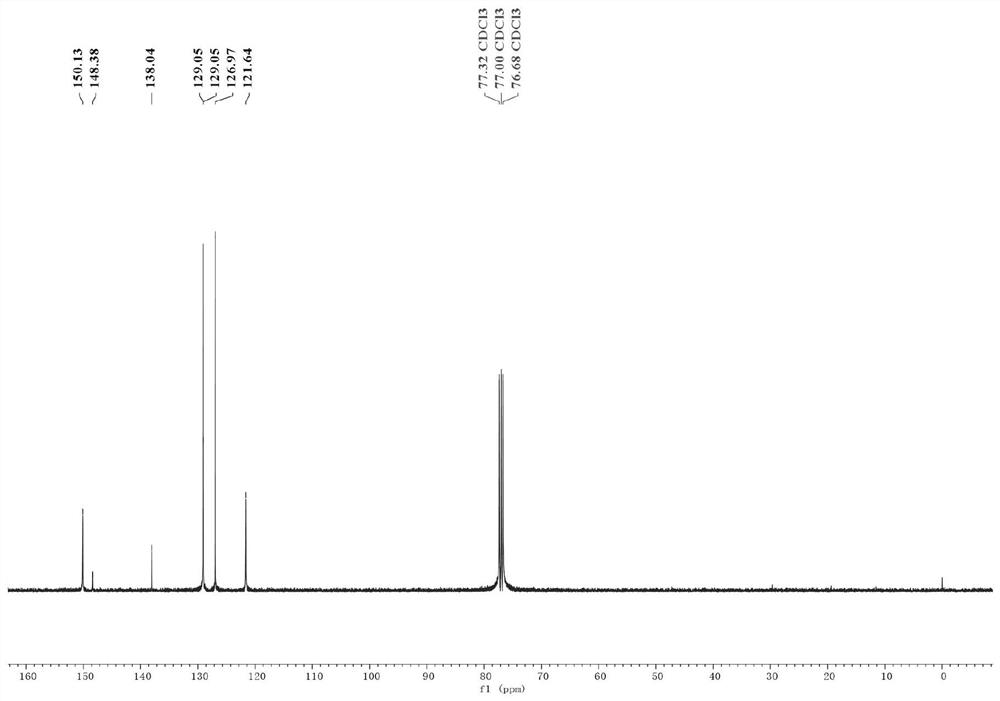
[0035] (1) Put the sealed tube equipped with a magnetic stirrer in an oven to dry for one hour, take it out and plug it with a rubber stopper while it is hot and insert a nitrogen balloon; then weigh magnesium chips (65.4mg, 1.5mmol, 3equiv. ) and lithium chloride (84.7mg, 2mmol, 4equiv.) and added to the sealed tube; then under reduced pressure, use an electric heating gun to heat the mixture of magnesium chips and lithium chloride (320 ° C, 3 minutes);
[0036] (2) After the mixture was cooled to room temperature, 2 mL of ultra-dry tetrahydrofuran was added thereto, and then the tube was sealed and replaced with nitrogen three times. Subsequently, triphenyl(pyridin-4-yl)phosphonium trifluoromethanesulfonate (244.7 mg, 1 mmol, 1 equiv.), bistriphenylphosphine palladium nickel dichloride (65.4 mg, 0.1 mmol , 20mol%), 1,10-phenanthroline-5,6-dione (21.0mg, 0.1mmol, 20mol%), bromobenzene (236.4mg, 1.5mmol, 3equiv.); the mixture was stirred at room temperature for 12 hours ;
...
Embodiment 2
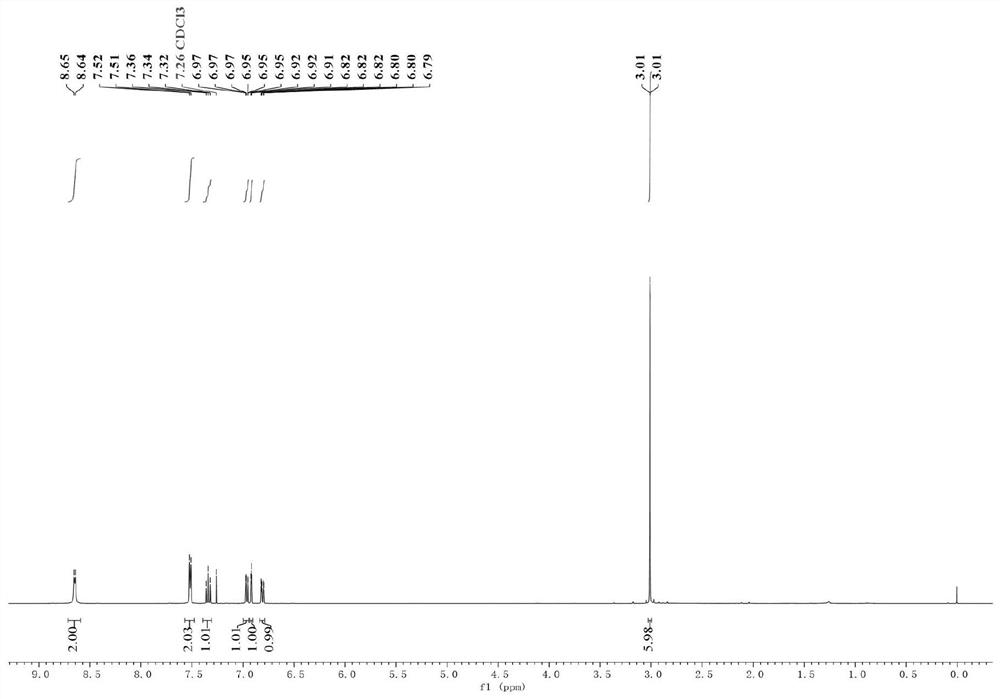
[0042] (1) Put the sealed tube equipped with a magnetic stirrer in an oven to dry for one hour, take it out and plug it with a rubber stopper while it is hot and insert a nitrogen balloon; then weigh magnesium chips (65.4mg, 1.5mmol, 3equiv. ) and lithium chloride (84.7mg, 2mmol, 4equiv.) and added to the sealed tube; then under reduced pressure, use an electric heating gun to heat the mixture of magnesium chips and lithium chloride (320 ° C, 3 minutes);
[0043] (2) When the mixture was cooled to room temperature, 2 mL of ultra-dry tetrahydrofuran was added to it, and then the sealed tube was replaced with nitrogen three times; then triphenyl(pyridin-4-yl)phosphonium trifluoromethanesulfonate was added to the sealed tube respectively (244.7mg, 1mmol, 1equiv.), bistriphenylphosphine palladium nickel dichloride (65.4mg, 0.1mmol, 20mol%), 1,10-phenanthroline-5,6-dione (21.0mg, 0.1 mmol, 20mol%), 3-bromo-N,N-dimethylaniline (300.4mg, 1.5mmol, 3equiv.); the mixture was stirred at ...
Embodiment 3
[0049] (1) Put the sealed tube equipped with a magnetic stirrer in an oven to dry for one hour, take it out and plug it with a rubber stopper while it is hot and insert a nitrogen balloon; then weigh magnesium chips (65.4mg, 1.5mmol, 3equiv. ) and lithium chloride (84.7mg, 2mmol, 4equiv.) and added to the sealed tube; then under reduced pressure, use an electric heating gun to heat the mixture of magnesium chips and lithium chloride (320 ° C, 3 minutes);
[0050] (2) When the mixture was lowered to room temperature, 2 mL of ultra-dry tetrahydrofuran was added thereto, and then the sealed tube was replaced with nitrogen three times; then (4-triphenylphosphonium quinoline) trifluoromethanesulfonate (4-triphenylphosphonium quinoline) triflate ( 269.6mg, 1mmol, 1equiv.), bistriphenylphosphine palladium nickel dichloride (65.4mg, 0.1mmol, 20mol%), 1,10-phenanthroline-5,6-dione (21.0mg, 0.1mmol , 20mol%), bromobenzene (236.4mg, 1.5mmol, 3equiv.); the mixture was stirred at room temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com