Mass spectrometry method for compound containing carbon-carbon double bonds
A carbon-carbon double bond and mass spectrometry analysis technology, applied in the field of mass spectrometry, can solve the problems of low reaction efficiency of methylation reagents, harsh reaction conditions, time-consuming and labor-consuming, etc., and achieve good substrate compatibility and functional group tolerance , The reaction conditions are simple and mild, and the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] A method for mass spectrometry analysis of compounds containing carbon-carbon double bonds, comprising the steps of:
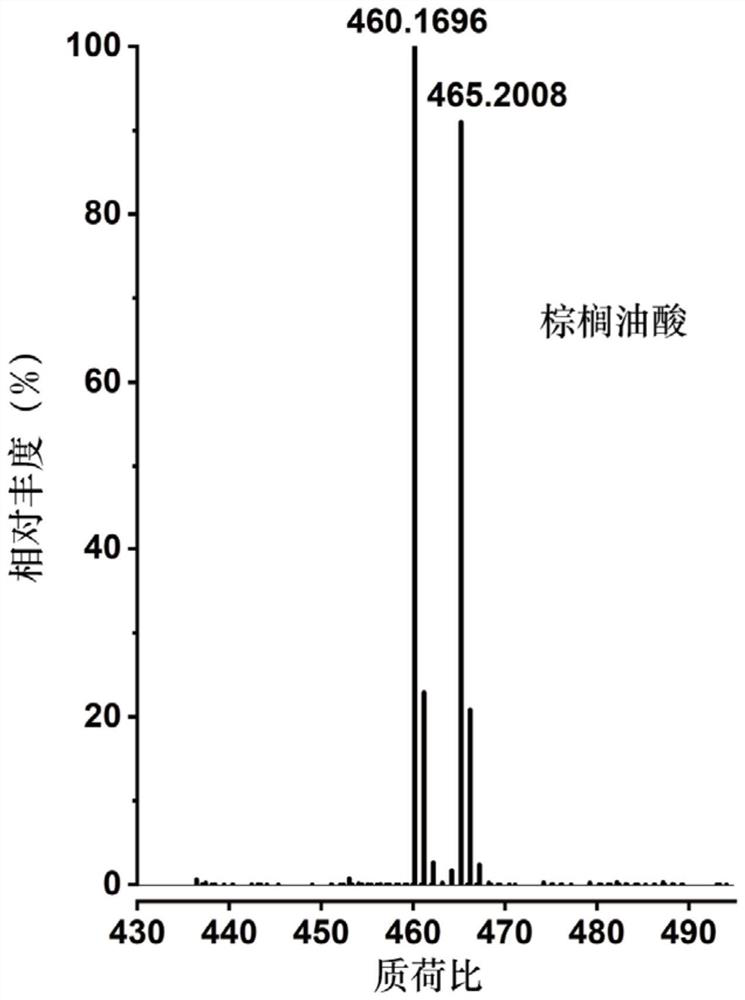
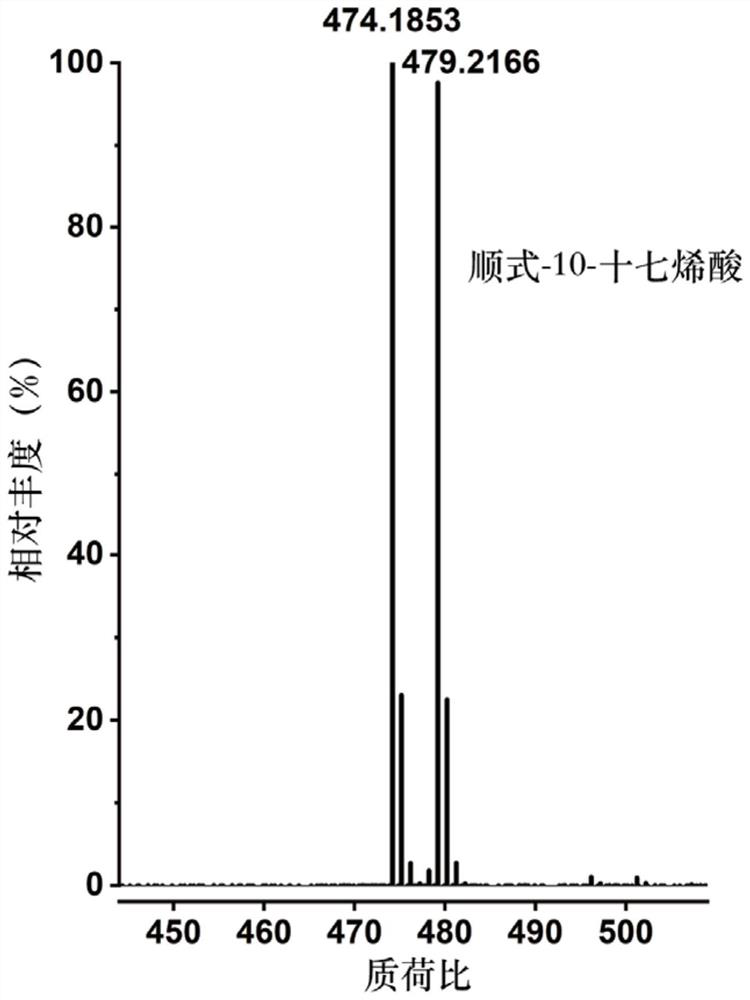
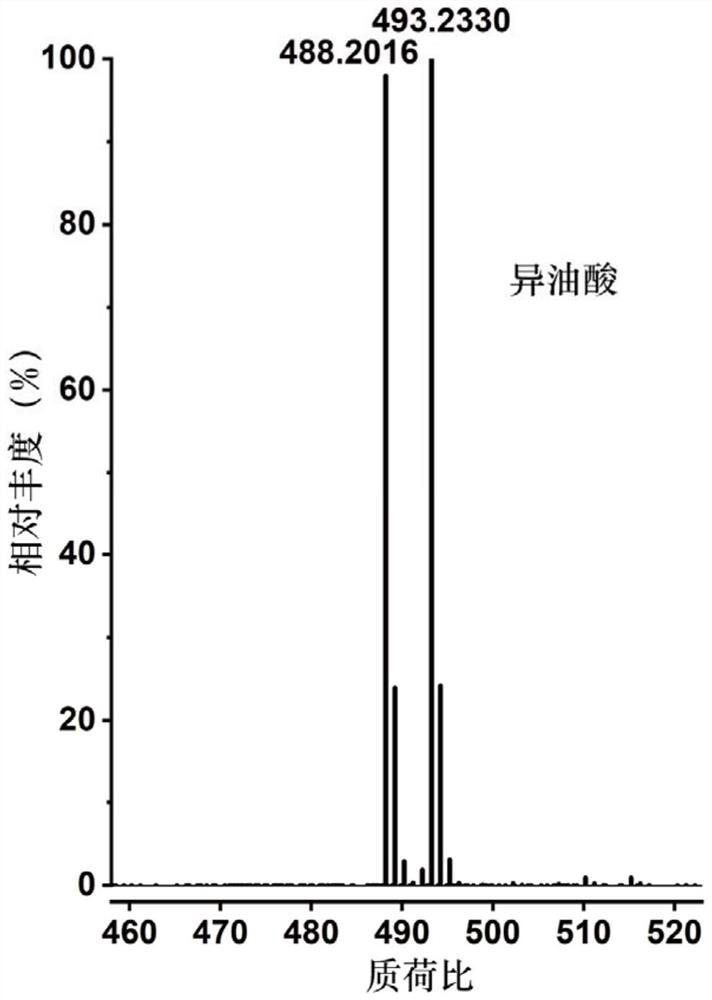
[0057] 1) respectively configure a series of compounds containing carbon-carbon double bonds (selected in this example: palmitoleic acid, cis-10-heptadecenoic acid, linoleic acid, oleic acid, linoleic acid, α-linolenic acid Acid, arachidonic acid, erucic acid, nervonic acid, oleyl alcohol, methyl erucate, but not limited to these compounds listed, can be fatty acids containing carbon-carbon double bonds, fatty alcohols containing carbon-carbon double bonds, containing Any one or more of fatty acid esters of carbon-carbon double bonds or terpenoids containing carbon-carbon double bonds) in dichloromethane, the concentration of each solution is 1 mg / mL;
[0058] 2) Take 10 μL of each of the above solutions in different derivatization bottles, and dry them with nitrogen for later use;
[0059] 3) Add 100 μL to each of the above 11 substrates after nitroge...
Embodiment 2
[0067] 1) Prepare dichloromethane solutions of oleic acid, linoleic acid, oleyl alcohol, and methyl erucate respectively, and the concentration of each solution is 1 mg / mL;
[0068] 2) Take 10 μL of each of the above-mentioned solutions in different derivatization bottles, and take 2 parts of the dichloromethane solution of each compound at the same time, and dry them with nitrogen for later use;
[0069] 3) Add 100 μL of 1000 μg / mL [d 0 ]-IPy 2 BF 4 Dichloromethane solution, add 100 μL of 1000 μg / mL [d 10 ]-IPy 2 BF 4 The dichloromethane solution was then vortexed at room temperature for 1 minute, and then the labeling reaction was carried out at room temperature on a shaker for 2 hours;
[0070] 4) Add 100 μL sodium thiosulfate aqueous solution with a mass fraction of 10% to the above labeling reaction system to quench the labeling reaction, then collect the lower organic phase solution, and redissolve in 100 μL containing 0.1 wt% formic acid after drying with nitrogen....
Embodiment 3
[0078] The operation of quantitatively analyzing compounds containing carbon-carbon double bonds using the mass spectrometry method of the present invention is as follows:
[0079]1. Configure a series of compounds containing carbon-carbon double bonds (selected in this example: palmitoleic acid, cis-10-heptadenoic acid, linoleic acid, oleic acid, linoleic acid, α-linolenic acid, etc.) Acid, arachidonic acid, erucic acid, nervonic acid, oleyl alcohol, methyl erucate, but not limited to these compounds listed, can be fatty acids containing carbon-carbon double bonds, fatty alcohols containing carbon-carbon double bonds, containing Dichloromethane solution of fatty acid ester of carbon-carbon double bond or any one or more of terpenoids containing carbon-carbon double bond), the concentration of each solution is 10nmol / mL, take 500μL of each solution respectively In different derivatization bottles, dry with nitrogen for use;
[0080] 2. Add a) 500 μL of 1000 nmol / mL [d 0 ]-IP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com