Synthesis method of 4-3 (H) quinazolinone and derivative thereof
A quinazolinone and a synthesis method technology, applied in the field of biomedicine, can solve the problems of high toxicity of isocyanate, many reaction steps, easy carbonization and blackening of reactants, etc., and achieve the effects of good functional group tolerance and short reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
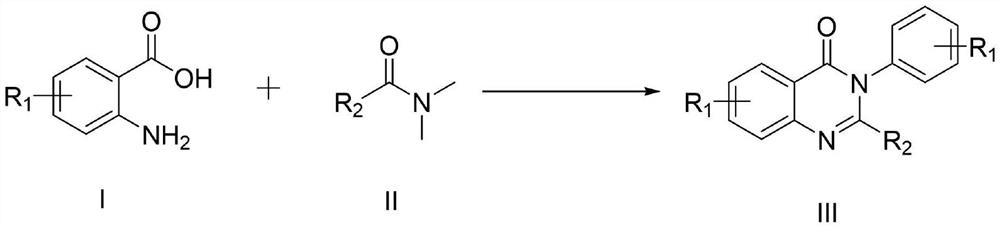
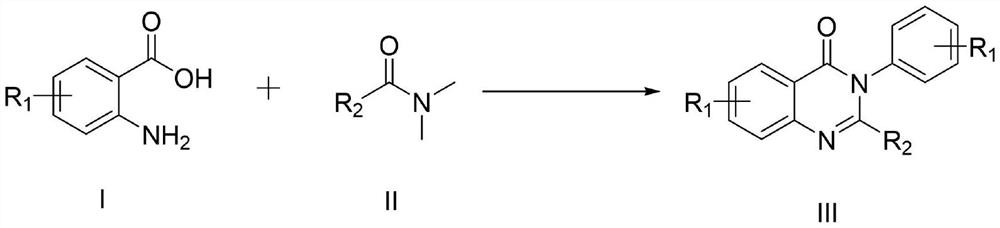
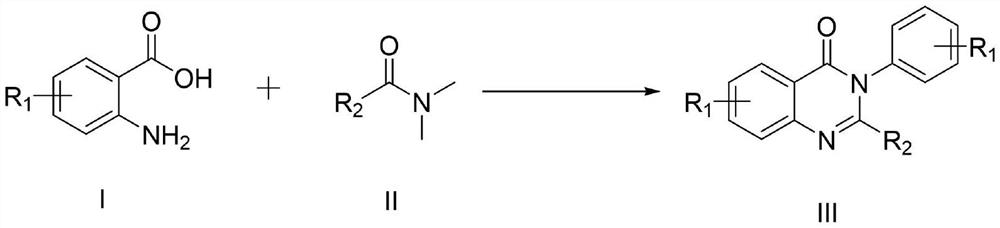
[0050] The general preparation steps that the present invention obtains are:
[0051] In a 10ml three-necked flask, add the compound of formula I, the compound of formula II and imidazole hydrochloride as a reaction catalyst, place the reaction in an oil bath at 150°C for heating, monitor the reaction process by TLC, add 30ml of distilled water after the reaction, and mix the obtained The mixture was extracted three times with 30ml ethyl acetate, and the combined layers were washed with H 2 O (50ml) wash, then brine (50ml), then anhydrous Na 2 SO 4 After drying, filtration and concentration under reduced pressure, the residue was purified by silica gel column chromatography (petroleum ether and ethyl acetate = 25:1 solvent), and recrystallized from ethyl acetate and n-hexane to obtain the target product.
[0052] In the initial experiment, the inventors carried out the reaction of cyclization of 2-amino-3-methylbenzoic acid (1b) and DMF (2a) to synthesize 4-(3H) quinazolinone ...
specific Embodiment
[0067] Embodiment 1 Synthetic 2, the general method (3a-3f) of 3-disubstituted quinazolinone derivatives
[0068] Into a 10ml three-necked flask were added 1a (0.41g, 3.0mmol, 1eq), imidazole hydrochloride (0.16g, 1.5mmol, 0.5eq) and N,N-dimethylformamide (2ml). The resulting solution was warmed to 150° C. and stirred at this temperature for 10 h, followed by TLC plate monitoring. When the reaction was complete, 30 ml of water was added, and the resulting mixture was extracted three times with 30 ml of ethyl acetate. with H 2 O (50ml) washed, then brine (50ml), then anhydrous Na 2 SO 4 After drying, filtering and concentrating under reduced pressure, the residue was purified by silica gel column chromatography and recrystallized from n-hexane and ethyl acetate to obtain the target product.
[0069] General method for the synthesis of 2,3-disubstituted quinazolinone derivatives (3g-3r)
[0070] A mixture of 1b (0.45g, 3.0mmol, 1eq), imidazole hydrochloride (0.16g, 1.5mmol, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com