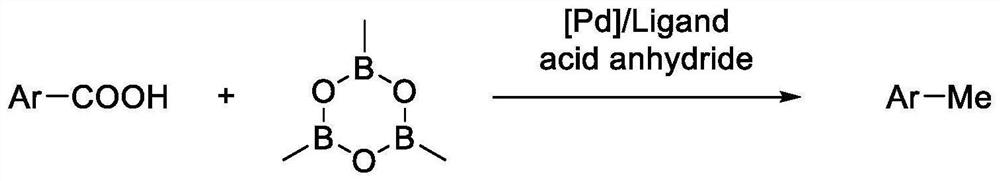
Method for preparing methyl (hetero) arene through decarbonylation coupling of (hetero) aryl formic acid and trimethylcyclotrioxane under catalysis of transition metal
A technology of trimethylcyclotrioxaborane and methyl heteroaromatic hydrocarbons, which is applied in the fields of organic synthesis and methyl aromatic hydrocarbon synthesis, can solve the problems of poor tolerance of reactive functional groups, expensive halogen-containing wastes, difficult separation and the like, and achieves the The effect of avoiding halogen-containing waste, easy column chromatography separation, good functional group tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
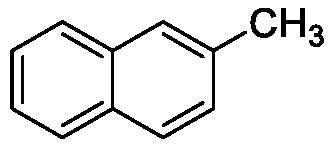
[0027] The structural formula of the target product is as follows:
[0028]
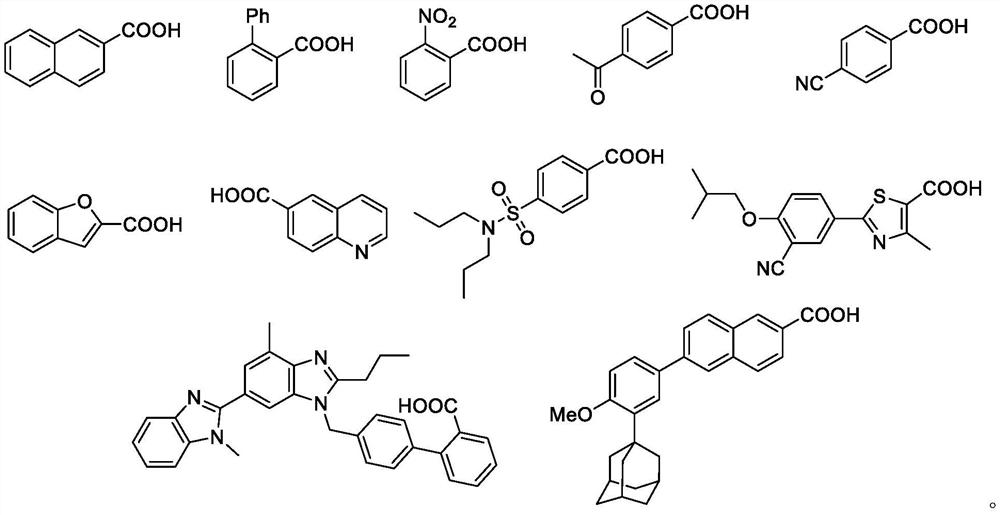
[0029] At room temperature, add 0.2 mmol of 2-naphthoic acid and 0.01 mmol of Pd(OAc) to a 50 mL reaction tube equipped with a magnetic stir bar 2 , 0.03mmol of XantPhos, after replacing the reaction tube with nitrogen, add 0.3mmol of Piv 2 O, 0.4 mmol of trimethylboroxine and 2 mL of 1,4-dioxane, after sealing the reaction system, place it in an oil bath heater with magnetic stirring, and react at 160°C for 24 hours . After the reaction was completed, dichloromethane was added for extraction and separation, and the product was obtained by column chromatography.
[0030] The product is a colorless liquid, the yield is 88%
[0031] 1 H NMR (400MHz, CDCl 3 )δ=2.53(s,3H),7.33(dd,J=8.4Hz,1.6,1H),7.39–7.48(m,2H),7.63(s,1H),7.75–7.78(m,2H),7.81 (d,J=8.0Hz,1H)ppm. 13 C NMR (101MHz, CDCl 3 )δ=21.9, 125.1, 126.0, 127.0, 127.4, 127.7, 127.8, 128.2, 131.8, 133.8, 135.6ppm.
Embodiment 2
[0033] The structural formula of the target product is as follows:
[0034]
[0035] The steps are the same as in Example 1, using o-phenylbenzoic acid as a raw material, and the reaction time is 12 hours.
[0036] The product was a pale yellow liquid with a yield of 82%.
[0037] 1 H NMR (400MHz, CDCl 3 ): δ=2.27(s,3H),7.22–7.28(m,3H),7.30–7.36(m,3H),7.37–7.62(m,3H)ppm; 13 C NMR (100MHz, CDCl 3 ): δ=20.6, 125.9, 126.9, 127.3, 127.4, 128.2, 128.9, 129.3, 129.9, 130.4, 135.5ppm.
Embodiment 3
[0039] The structural formula of the target product is as follows:
[0040]
[0041] Step is with embodiment 1, is raw material with o-nitrobenzoic acid.
[0042]The product was a yellow liquid with a yield of 84%.
[0043] 1 H NMR (400MHz, CDCl 3 ):δ=2.61(s,3H),7.30–7.39(m,2H),7.50(t,J=7.6Hz,1H),7.97(d,J=8.4Hz,1H)ppm. 13 C NMR (100MHz, CDCl 3 ): δ=20.6, 124.8, 127.0, 132.9, 133.1, 133.7, 149.5ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com