Application of nitrogen-containing heterocyclic mercaptan cuprous compound in photocatalytic reaction of carbonyl compound
A carbonyl compound and photocatalytic reaction technology, which is applied in the direction of organic compound/hydride/coordination complex catalyst, catalytic reaction, hydroxyl compound preparation, etc., can solve the problems of limited scope and difficulty in popularization and application, and achieve low price, Good catalytic effect and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Nitrogen-containing heterocyclic mercaptan monovalent copper compound catalyzes the hydrogen transfer reaction of acetophenone
[0031]
[0032] Add 0.2mmoL of acetophenone, 0.1mmoLNaOH, 0.3mmoLHEH and 4mg of nitrogen-containing heterocyclic thiol monovalent copper compound into a dry reflux reaction tube with a magnetic stirrer, and then add 5mL of anhydrous The mixed solution of isopropanol and acetonitrile was stirred for reaction. During the reaction with N 2 Replaced 3 times, using blue LEDs light as the light source of the catalytic reaction, reacted for 24 h, added 5 mL of water after completion, extracted with 3×5 mL of ethyl acetate, combined the organic phase, dried the organic phase with anhydrous magnesium sulfate, filtered, and the filtrate After concentrated by rotary evaporation, the target product was obtained by separation by silica gel column chromatography, and the yield of the target product was 98%.
[0033] The nuclear magnetic s...
Embodiment 2
[0034] Example 2 Nitrogen-containing heterocyclic mercaptan monovalent copper compound catalyzes the hydrogen transfer reaction of p-methylacetophenone
[0035]
[0036] Add 1mmoL p-methylacetophenone, 0.1mmoLNaOH, 0.3mmoLHEH and 4mg nitrogen-containing heterocyclic thiol monovalent copper compound into a dry reflux reaction tube with a magnetic stirrer, then add 5 mL, the volume ratio is 3:1 A mixed solution of anhydrous isopropanol and acetonitrile was stirred for reaction. During the reaction process, replace with helium 3 times, use the blue LEDs light as the light source of the catalytic reaction, stir the reaction for 36 hours, after the end, add 5mL of water, extract with 3×5mL ethyl acetate, combine the organic phases, and dry with anhydrous magnesium sulfate , filtered, and the filtrate was concentrated by rotary evaporation, and then separated by silica gel column chromatography to obtain the target product, and the yield of the target product was 99%.
[0037] T...
Embodiment 3
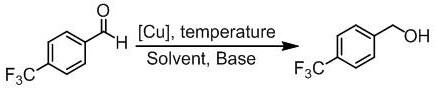
[0038] Example 3 Nitrogen-containing heterocyclic mercaptan monovalent copper compound catalyzes the hydrogen transfer reaction of 4-fluoroacetophenone
[0039]
[0040] Add 1mmoL of 4-fluoroacetophenone, 0.1mmoLNaOH, 0.3mmoLHEH and 4mg of nitrogen-containing heterocyclic thiol monovalent copper compound into a dry reflux reaction tube with a magnetic stirrer, and then add 5mL of non- The mixed solution of water isopropanol and acetonitrile was stirred for reaction. During the reaction process, neon gas was used to replace 3 times, blue LEDs were used as the light source for the catalytic reaction, and the reaction was stirred for 30 hours. After the reaction was completed, 5 mL of water was added, extracted with 3×5 mL of ethyl acetate, and the organic phases were combined and dried with anhydrous magnesium sulfate. , filtered, and after the filtrate was concentrated by rotary evaporation, the target product was obtained by silica gel column chromatography, and the yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com