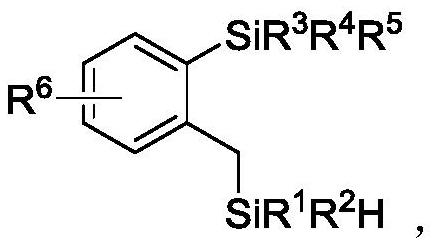
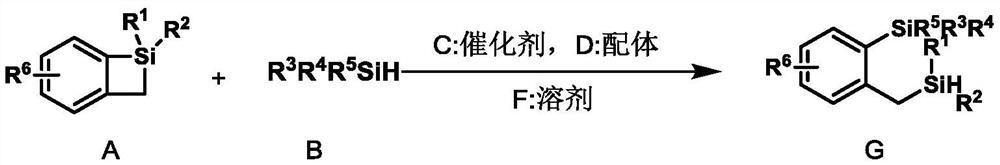
A kind of double silicon compound, its preparation method and application
A technology for silicon compounds and silanes, which is applied to organic silicon compounds, chemical instruments and methods, and compounds of elements of Group 4/14 of the periodic table. , good substrate universality, good tolerability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
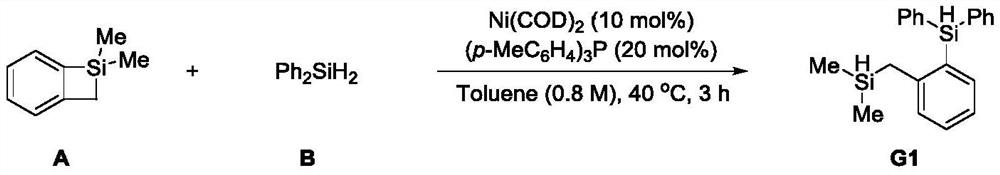
Embodiment 1
[0035]
[0036] Under the protection of inert gas, Ni(COD) was added to a dry 10.0mL reaction tube equipped with a magnetic stirrer 2 (5.5mg, 0.02mmol, 10mol%), (p-MeC 6 h 4 ) 3 P (12.2 mg, 0.04 mmol, 20 mol%), dried toluene (0.25 mL), A (29.6 mg, 0.2 mmol), diphenylsilane B (73.6 mg, 0.4 mmol, 2 equiv.). Seal the tube tightly and heat at 40°C for three hours. Then it was cooled to room temperature, and the reaction solution was suction-filtered with celite, and 30 ml of ethyl acetate was used as eluent. After the filtrate was spin-dried under reduced pressure, it was purified by a silica gel column, and the eluent was petroleum ether to obtain G1 (60 mg of colorless oily liquid, yield 90%). 1 H NMR (400MHz, CDCl 3 ,25℃)δ7.57–7.56(m,2H),7.56–7.54(m,2H),7.46–7.41(m,2H),7.40–7.35(m,4H),7.35–7.29(m,2H) ,7.15–7.10(m,1H),7.07(td,J=7.4,1.1Hz,1H),5.64(s,1H),3.92–3.86(m,1H),2.33(d,J=3.4Hz,2H ),0.02(d,J=3.7Hz,6H). 13 C NMR (101MHz, CDCl 3 ,25℃) δ147.4, 137.3, 136.1, 133.7, ...
Embodiment 3
[0038]
[0039] Under the protection of inert gas, Ni(COD) was added to a dry 10.0mL reaction tube equipped with a magnetic stirrer 2 (5.5mg, 0.02mmol, 10mol%), (p-MeC 6 h 4 ) 3 P (12.2mg, 0.04mmol, 20mol%), dry toluene (0.25mL), A (29.6mg, 0.2mmol), phenyl(m-fluorophenyl)silane B (80.8mg, 0.4mmol, 2equiv.). Seal the tube tightly and heat at 40°C for three hours. Then it was cooled to room temperature, and the reaction solution was suction-filtered with celite, and 30 ml of ethyl acetate was used as eluent. After the filtrate was spin-dried under reduced pressure, it was purified by a silica gel column, and the eluent was petroleum ether to obtain G3 (63 mg of colorless oily liquid, yield 90%). 1 H NMR (400MHz, CDCl 3 ,25℃)δ7.56–7.51(m,2H),7.47–7.42(m,1H),7.41–7.27(m,6H),7.24–7.20(m,1H),7.15–7.05(m,3H) ,5.63(s,1H),3.95–3.83(m,1H),2.31(d,J=3.4Hz,2H),0.02(d,J=3.7Hz,6H). 13 C NMR (101MHz, CDCl 3 ,25℃)δ162.79(d,J=248.6Hz),147.5,137.3,136.9(d,J=4.4Hz),136.0,132.9,131.7(d...
Embodiment 4
[0041]
[0042] Under the protection of inert gas, Ni(COD) was added to a dry 10.0mL reaction tube equipped with a magnetic stirrer 2 (5.5mg, 0.02mmol, 10mol%), (p-MeC 6 h 4 ) 3 P (12.2 mg, 0.04 mmol, 20 mol%), dry toluene (0.25 mL), A (29.6 mg, 0.2 mmol), phenyl (biphenyl) silane (104 mg, 0.4 mmol, 2 equiv.). Seal tightly Tubes were then heated at 40 °C for three hours. Then it was cooled to room temperature, and the reaction solution was suction-filtered with celite, and 30 ml of ethyl acetate was used as eluent. After the filtrate was spin-dried under reduced pressure, it was purified with a silica gel column, and the eluent was petroleum ether to obtain G6 (60 mg of colorless oily liquid, yield 74%). 1 H NMR (400MHz, CDCl 3 ,25℃)δ7.66–7.56(m,8H),7.50–7.30(m,8H),7.18–7.02(m,2H),5.68(s,1H),4.10–3.59(m,1H),2.36 (d,J=3.4Hz,2H),0.03(d,J=3.7Hz,6H). 13 C NMR (101MHz, CDCl 3 ,25℃).δ147.5,142.5,141.1,137.4,136.6,136.1,133.7,132.4,130.8,130.3,129.9,128.9,128.7,128.2,127.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com