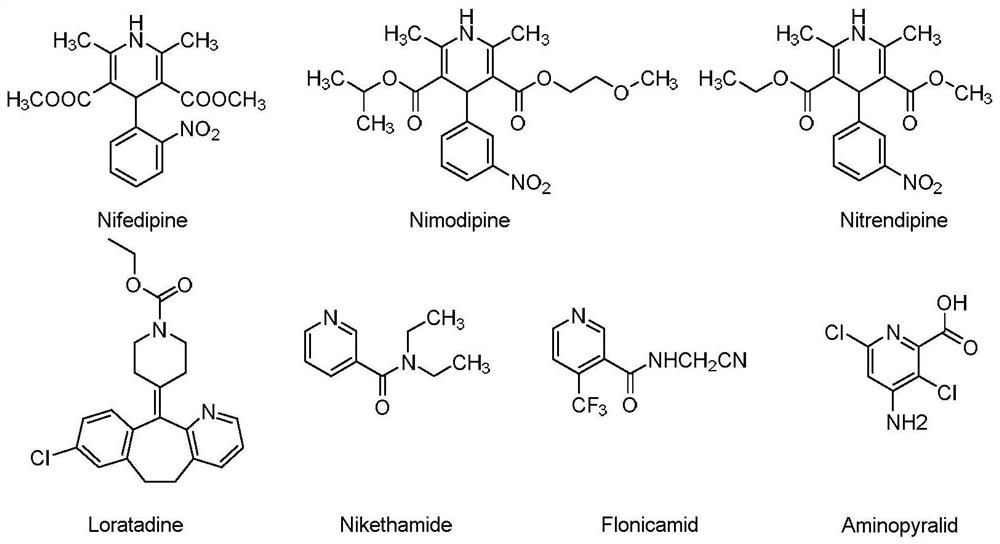
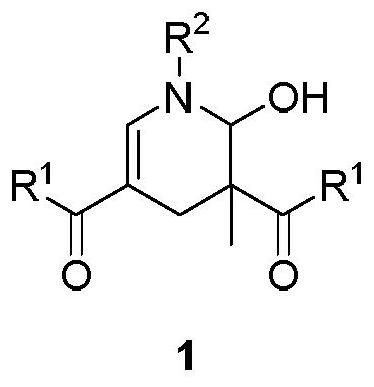
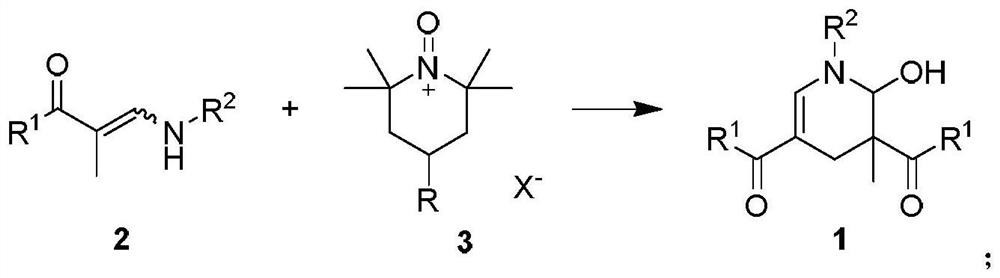
2-hydroxypyridine compound and its synthesis method
A technology of hydroxypyridine and synthesis method, applied in the direction of organic chemistry, organic chemistry, etc., to achieve the effects of wide applicability, easy price, and simple and mild synthesis reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] Weigh 3-(benzylamino)-1-(4-chlorophenyl)-2-methylprop-2-en-1-one 2a (0.6mmol) into a 25mL Schlenk reaction flask, add 6mL of DCM, at 40°C Stir in an oil bath for 2 minutes, add Tempo salt 3a (0.6 mmol), and react for 1 h. After the reaction, the mixture was cooled to room temperature, extracted with dichloromethane and water, the organic phase was collected, dried over anhydrous sodium sulfate, filtered, and the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum Ether (60-90°C) / ethyl acetate, v / v=10:1), the target product 1a-α (75mg, yield 58%), 1a-β (23mg, yield 17%) was obtained as colorless oil , dr:77:23. The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0047]
[0048] Weigh 3-(benzylamino)-1-(4-methylphenyl)-2-methylprop-2-en-1-one 2b (0.6mmol) into a 25mL Schlenk reaction flask, add 2mL of toluene, and Stir in an oil bath for 2 minutes, add Tempo salt 3b (0.7 mmol), and react for 0.2 h. After the reaction, the mixture was cooled to room temperature, extracted with dichloromethane and water, the organic phase was collected, dried over anhydrous sodium sulfate, filtered, and the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum Ether (60-90°C) / ethyl acetate, v / v=10:1), the target product 1b-α (73mg, yield 55%), 1b-β (25mg, yield 20%) was obtained as colorless oil , dr:74:26. The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 3
[0050]
[0051]Sequentially weigh 3-(benzylamino)-1-(4-methoxyphenyl)-2-methylprop-2-en-1-one 1-ketone-1-phenyl-3-benzylamino-2 - Propylene 2c (0.6mmol) in a 25mL Schlenk reaction flask, add DMSO 2mL, stir in an oil bath at 80°C for 2 minutes, add Tempo salt 3c (0.6mmol), and react for 0.5h After the reaction is over, the mixture is cooled to At room temperature, dichloromethane and water were extracted, the organic phase was collected, dried over anhydrous sodium sulfate, filtered, and the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / Ethyl acetate, v / v=10:1), the target product 1c (112 mg, yield 80%, dr:95:5) was obtained as a colorless oil. The target product was confirmed by NMR and high-resolution mass spectrometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com