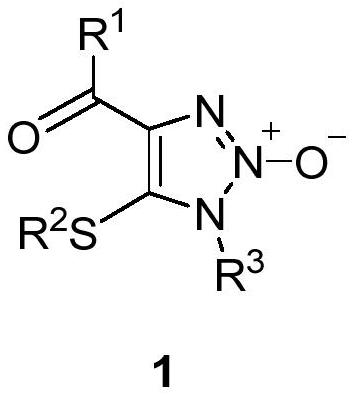
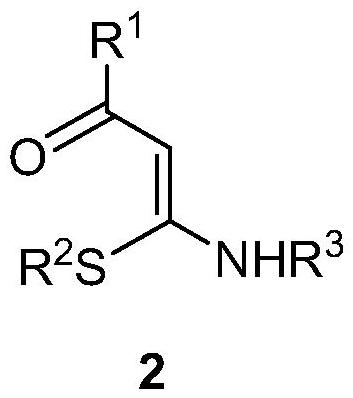
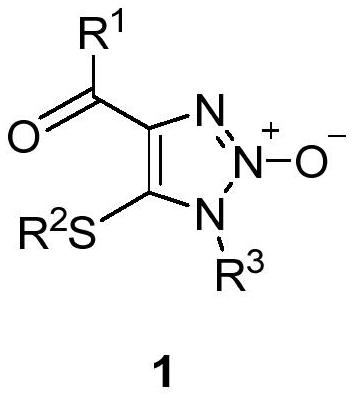
1, 2, 3-triazole-2-oxide and preparation method thereof
A technology of oxides and compounds, which is applied in the field of 1,2,3-triazole-2-oxide and its preparation, can solve the problems of limited substrate range and harsh reaction conditions, and achieve simple operation, easy product, The effect of a wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] In a 25mL sealed tube, add enaminone compound 2a (104mg, 0.5mmol), 4A molecular sieve 100mg, CuCl 2 (7mg, 0.05mmol), add 5mL MeCN, tert-butyl nitrite 3 (180μL, 1.5mmol) in an argon atmosphere, react at 80°C for 3h, and separate directly by column chromatography after the reaction (eluent, petroleum ether (60-90 ℃) / ethyl acetate=2:1, v / v), to obtain a light yellow solid product 1a (83 mg, yield 66%), melting point 82-83 ℃, the target product was determined by NMR and high-resolution mass spectrometry, the result as follows:
[0035] 1 H NMR (400MHz, CDCl 3 )δ8.15(m,2H,aromatic CH),7.57 and 7.46(t each,J=7.4 and 7.7Hz,1:2H,aromatic CH),3.92 and 2.58(s each,3:3H,2×CH 3 ). 13 C{ 1 H}NMR (100MHz, CDCl 3 )δ183.9(Cq,CO),136.6,136.5,and 128.51(Cq),133.5,130.5,and 128.45(aromatic CH),32.0 and 19.1(2×CH 3 ).HRMS(EI)m / z calcd for C 11 h 12 N 3 o 2 S[M+H] + :250.0650; Found: 250.0656.
Embodiment 2
[0037] The difference from Example 1 is that the solvent is dichloromethane to obtain the target product 1a (35 mg, yield 28%).
Embodiment 3
[0039] The difference from Example 1 is that the solvent is toluene, and the target product 1a (50 mg, yield 40%) is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com