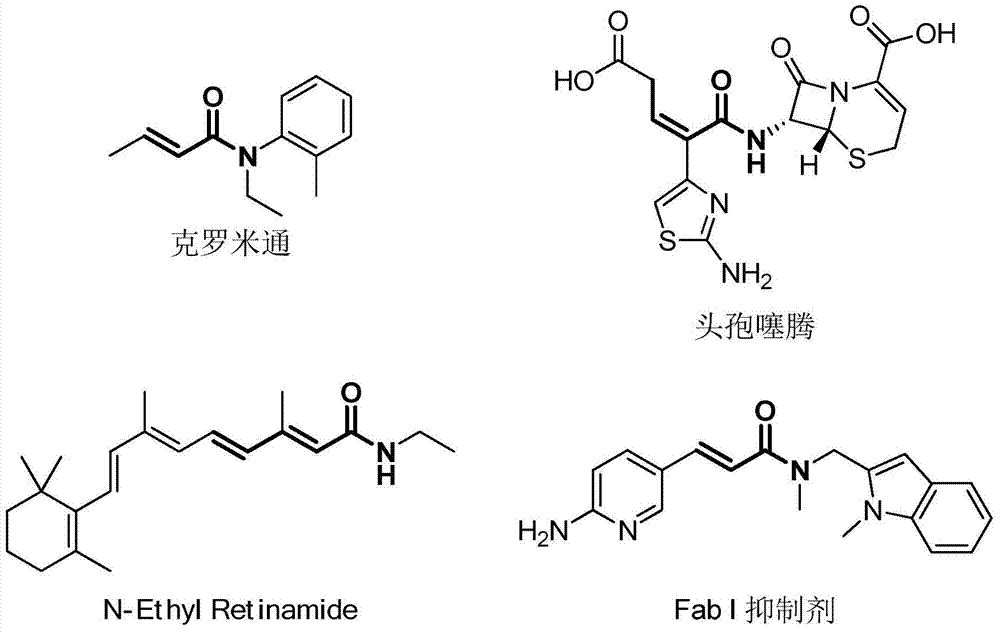
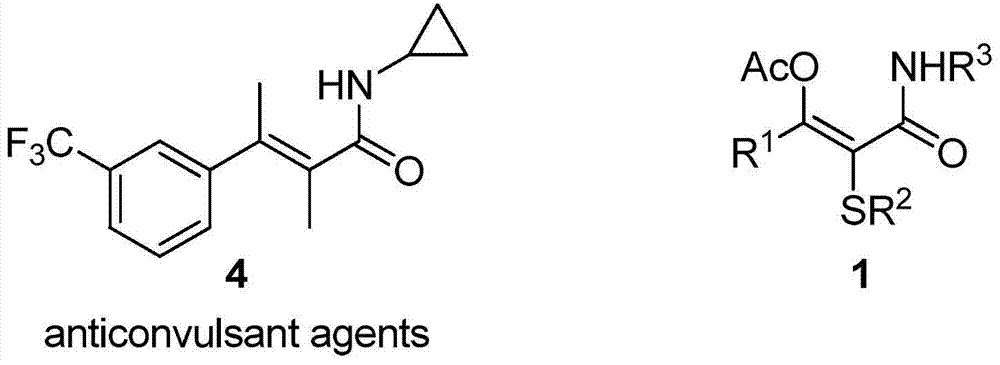
2-Alkylthioenamide derivatives and synthetic method thereof
A technology of alkylthioenamide and alkylthio, which is applied in the field of α, β-unsaturated amide compound 2-alkylthioenamide derivatives and its synthesis, which can solve the problem of weak atom economy, numerous preparation steps, Raw materials need to be activated, etc., to achieve good stereoselectivity and functional group diversity, easy to obtain raw materials, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
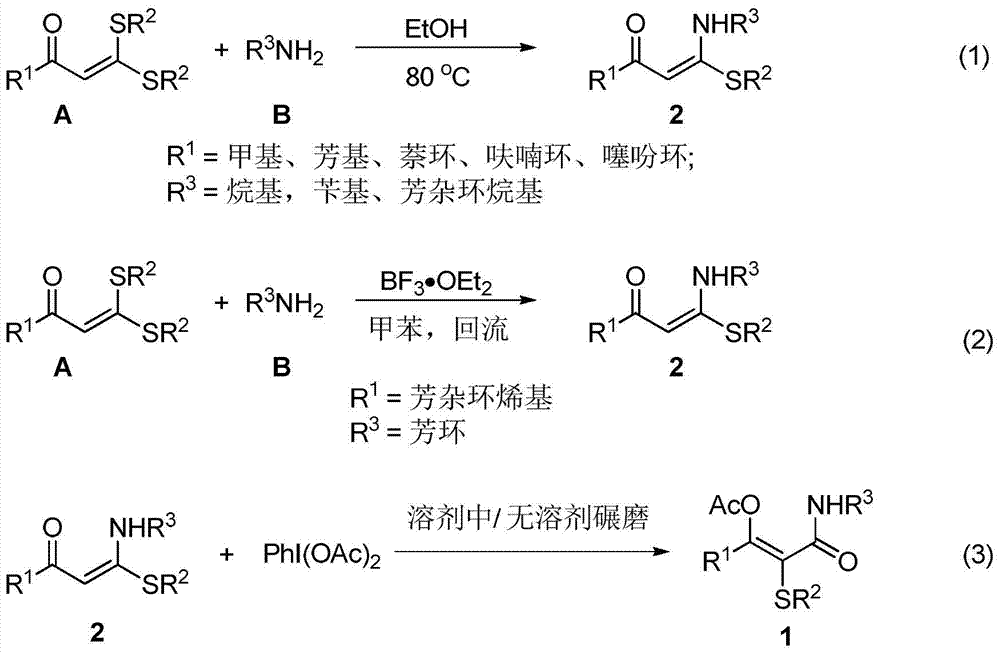
Image
Examples
Embodiment 1
[0040]
[0041] Weigh 1-thiomethyl-1-benzyl-1-en-3-one 2a (0.5mmol) and iodobenzene acetate (0.75mmol) in a 25mL Schlenk reaction flask, add DCE solvent 5mL under air, at 25 The reaction was stirred at °C for 2 hours. After the reaction, the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / ethyl acetate, v / v=20:1) to obtain the white solid target Product 1a (130 mg, yield 76%). The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0043] The reaction steps and operations are the same as in Example 1, except that the solvent is water. The reaction was stopped, and the target product 1a (40 mg, yield 23%) was obtained after post-processing.
Embodiment 3
[0045]The reaction steps and operations are the same as in Example 1, except that the temperature is 80°C. The reaction was stopped, and the target product 1a (99 mg, yield 58%) was obtained after post-treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com