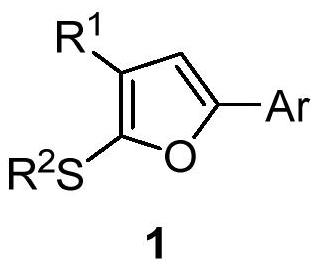
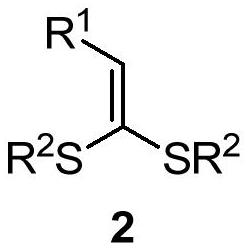
Synthesis method of 2-alkylthio polysubstituted furan derivative
The technology of a furan derivative and a synthesis method is applied in the field of synthesis of 2-alkylthio polysubstituted furan derivatives, can solve problems such as complicated reaction steps and the like, achieves low cost, mild reaction conditions, and cheap and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] In a 25 mL schlenk tube, add 3,3-dimethylthio-2-propen-1-one 2a (0.5 mmol), acetophenone 3a (1.0 mmol), cuprous iodide (10 mol%) sequentially under argon , di-tert-butyl peroxide (1.0 mmol) and 5.0 mL of benzotrifluoride, stirred at 100° C. for 20 hours. After cooling to room temperature, the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / ethyl acetate, v / v=70:1) to obtain the target product 1a (110 mg, yield 75%). The target product was confirmed by NMR and high-resolution mass spectrometry.
[0034] Typical Compound Characterization Data
[0035] 2-Alkylthio polysubstituted furan derivatives (1a), light yellow oily liquid. 1 H NMR (400MHz, CDCl 3 )δ7.76(d, J=7.3Hz, 2H), 7.54(d, J=7.5Hz, 2H), 7.47(d, J=7.3Hz, 1H), 7.41(t, J=7.4Hz, 2H) ,7.31(t,J=7.7Hz,2H),7.21(t,J=7.4Hz,1H),6.86(s,1H),2.61(s,3H). 13 C{ 1 H}NMR (100MHz, CDCl 3 )δ189.3, 158.2, 154.1, 1...
Embodiment 2
[0037] The reaction steps and operations are the same as in Example 1, except that the molar ratio of 2a and 3a is 1:1.5. The reaction was stopped, and the target product 1a (103 mg, yield 70%) was obtained after post-treatment.
Embodiment 3
[0039] Reaction steps and operation are with embodiment 1, and difference with embodiment 1 is that catalyst is CuCl 2 . The reaction was stopped, and the target product 1a (88 mg, yield 60%) was obtained after post-treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com