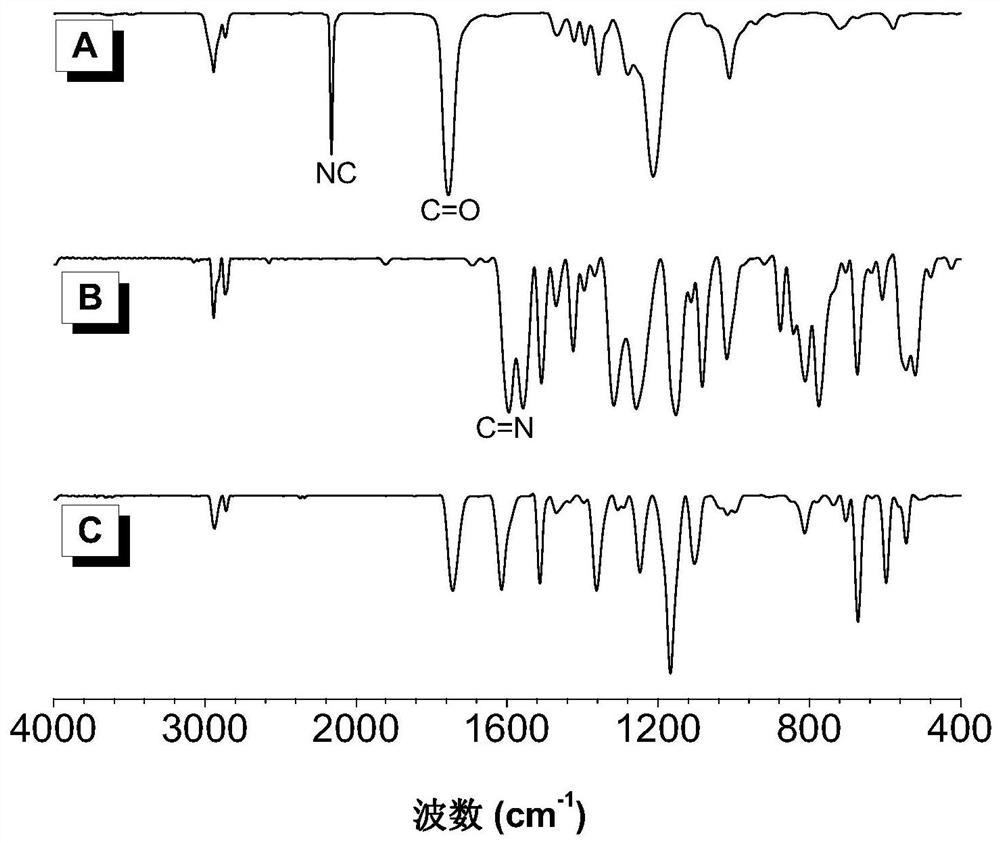
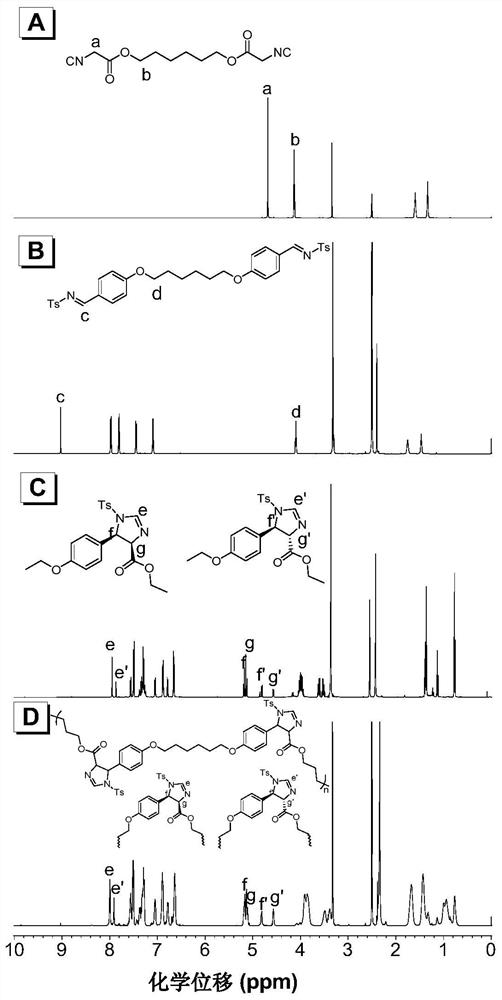
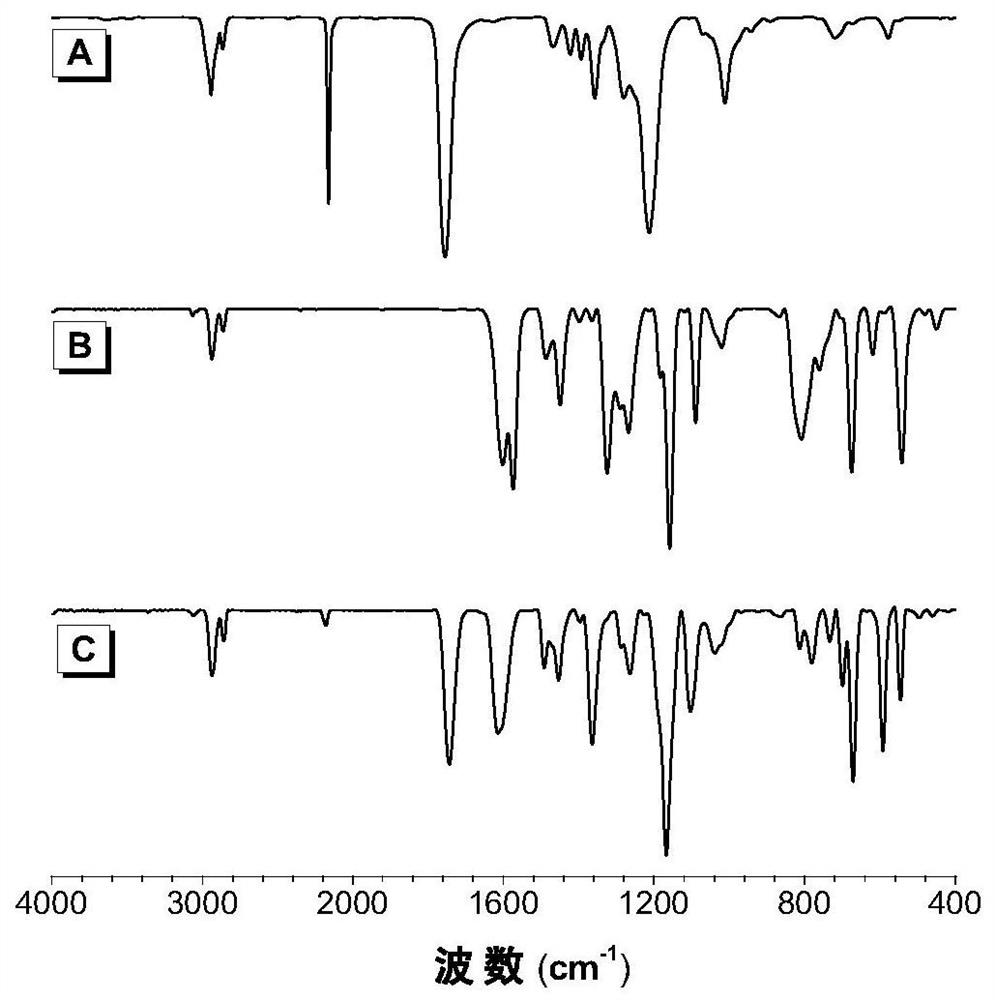
A kind of polyimidazoline compound and preparation method thereof
A polyimidazoline compound technology, applied in the field of polyimidazoline compounds and their preparation, can solve the problems of blank polymer synthesis methods, etc., and achieve the effects of high polymerization efficiency, high thermal stability, and good functional group tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] (1) The synthetic method of the first monomer binary isocyano compound 1a is as follows,
[0057]
[0058] (2) The synthetic method of the second monomer disulfonimide compound 2a is as follows,
[0059]
[0060] (3) Polymerization of isocyano-sulfonimide to prepare polyimidazoline compounds P1a2a
[0061]
[0062] Add 50.5 mg (0.20 mmol) of the first monomer, 126.6 mg (0.20 mmol) of the second monomer, 2.0 mg (0.02 mmol) of cuprous chloride and 10.5 mg (0.04 mmol) of triphenylphosphine into a 10 mL polymerization tube, The nitrogen gas was replaced three times, 2 mL of dichloromethane was added, and after the monomer was completely dissolved, the temperature was raised to 40°C. React for 8 hours. After the reaction solution was diluted with 2 mL of dichloromethane, it was added dropwise into 150 mL of rapidly stirring n-hexane through a cotton filter to obtain a white flocculent precipitate. Stand overnight, filter and dry to obtain the target polymer.
[...
Embodiment 2~3
[0070] Examples 2-3 investigated the influence of triphenylphosphine in the catalyst on this polymerization reaction. The preparation of the polymer monomer was the same as in Example 1. The reaction conditions and results of step (3) are shown in Table 1.
[0071] Table 1 Effect of triphenylphosphine on polymerization of monomers 1a and 2a a
[0072]
[0073] a Reaction in dichloromethane under nitrogen for 4h; T=40°C; [M] 0 = 100 mM.
[0074] b Determined by GPC, using linear polymethyl methacrylate as a calibration substance and DMF as a mobile phase.
[0075] By comparison, we found that the addition of triphenylphosphine greatly promoted the occurrence of the polymerization reaction. Under the same conditions, the polymerization reaction with addition of triphenylphosphine resulted in higher yield and higher molecular weight.
Embodiment 4~7
[0077] Examples 4-7 investigated the influence of different solvents on the reaction conditions. The preparation of the polymerized monomers is the same as in Example 1. The reaction conditions and results of step (3) are shown in Table 2.
[0078] Table 2 The influence of solvent on the polymerization of monomer 1a and 2a a
[0079]
[0080] a Reaction in different solvents under nitrogen for 4h; T=40°C; [M] 0 = 100 mM.
[0081] b Determined by GPC, using linear polymethyl methacrylate as a calibration substance and DMF as a mobile phase.
[0082] As can be seen in Table 2, DMF is not conducive to the occurrence of the polymerization reaction, and other solvents such as DCM, THF, chloroform, etc. can well occur the polymerization reaction. Considering productive rate, weight average molecular weight (M w ), the polydispersity coefficient We choose dichloromethane as the optimal conditions for the polymerization reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com