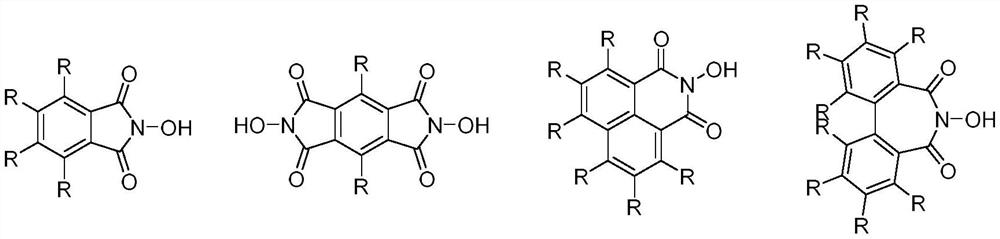
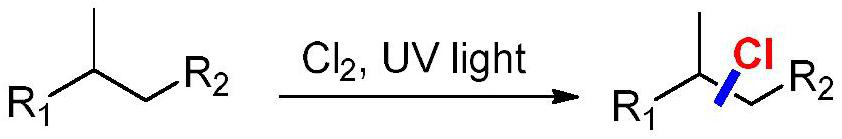Method for synthesizing halogenated alkane by high-selectivity halogenated saturated carbon-hydrogen bonds
A halogenated alkanes, high-selectivity technology, applied in the field of drug synthesis, can solve the problems of unsuitable industrial production, danger, cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Take isoamyl benzoate (96.0mg, 0.5mmol, 1.0eq), Et 4 NBF 4 (217.0mg, 1.0mmol, 2.0eq) and NHPI (16.3mg, 0.1mmol, 20mol%) in a 10.0mL three-necked bottle; add CH 3 CN (8.0mL), stirred to dissolve; add hydrochloric acid (aq.37%, 2.5mmol, 5.0eq); insert electrodes (the anode is graphite felt, the cathode is platinum sheet) respectively on the left and right sides of the three-neck flask, energize and The reaction was carried out at room temperature under constant current for 24 hours at a current of 10 mA. After the reaction was completed, the solvent was reclaimed by distillation, extracted with 2M NaOH aqueous solution and dichloromethane (3 × 5mL), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and after the solvent was reclaimed by distillation, the product was separated by column chromatography (yield: 89%).
Embodiment 2
[0029]
[0030] Take isoamyl benzoate (96.0mg, 0.5mmol, 1.0eq), Et 4 NBF 4 (2.17mg, 0.01mmol, 0.02eq) and NHPI (1.63mg, 0.01mmol, 20mol%) in a 10.0mL three-necked bottle; add CH 3 CN (8.0mL), stirred to dissolve; add hydrochloric acid (aq.37%, 2.5mmol, 5.0eq); insert electrodes (the anode is graphite felt, the cathode is platinum sheet) respectively on the left and right sides of the three-neck flask, energize and The reaction was carried out at room temperature under constant current for 24 hours at a current of 10 mA. After the reaction was completed, the solvent was reclaimed by distillation, extracted with 2M NaOH aqueous solution and dichloromethane (3 × 5mL), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and after the solvent was reclaimed by distillation, the product was separated by column chromatography (yield: 48%).
Embodiment 3
[0032]
[0033] Take isoamyl benzoate (96.0mg, 0.5mmol, 1.0eq), Et 4 NBF 4 (217.0mg, 1.0mmol, 2.0eq) and NHPI (163mg, 1.0mmol, 20mol%) in a 10.0mL three-necked bottle; add CH 3 CN (8.0mL), stirring to dissolve; add HCl (aq.37%, 2.5mmol, 5.0eq); insert electrodes (anode is graphite felt, cathode is platinum sheet) respectively on the left and right sides of the three-neck flask, and electrify and The reaction was carried out at room temperature under constant current for 24 hours at a current of 10 mA. After the reaction was completed, the solvent was reclaimed by distillation, extracted with 2M NaOH aqueous solution and dichloromethane (3 × 5mL), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and after the solvent was reclaimed by distillation, the product was separated by column chromatography (yield: 91%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com