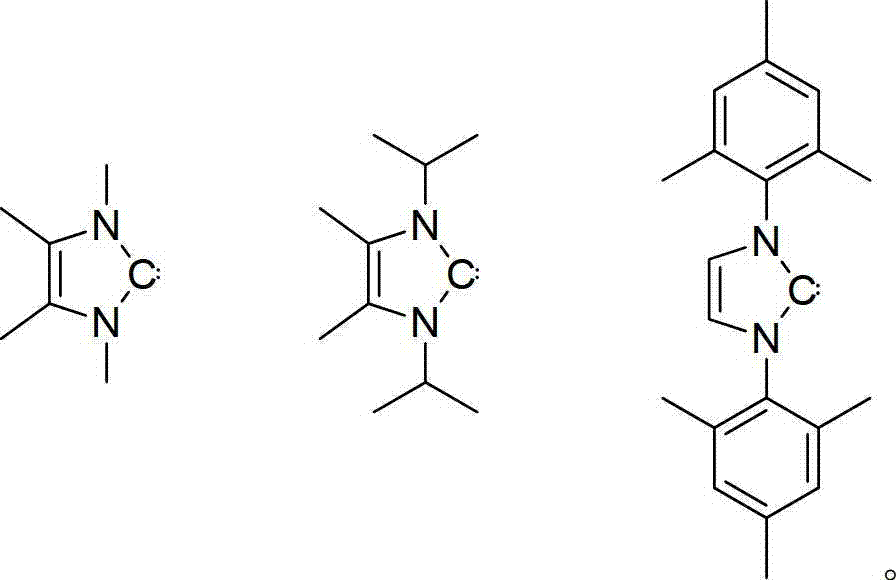
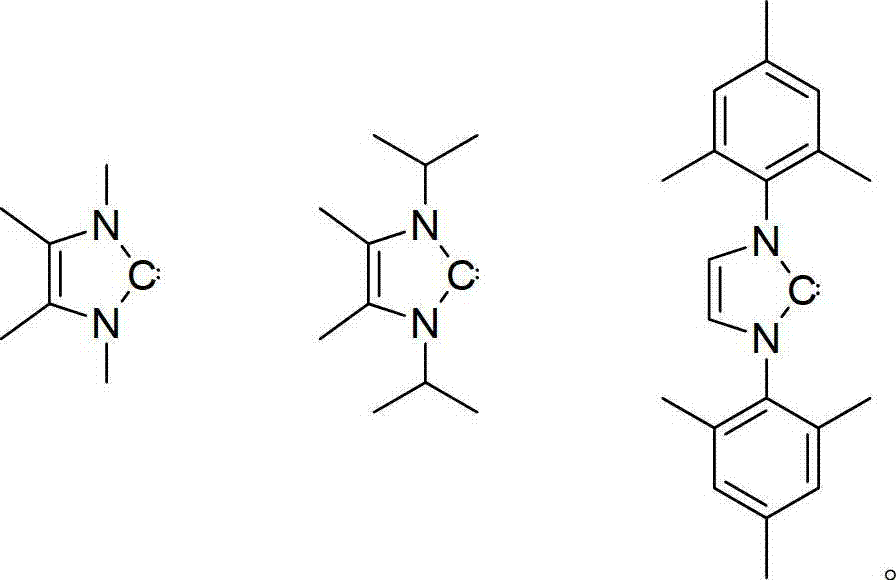
Method for catalyzing alcohol dehydrogenation silicon alkylation by using azacyclo-cabbeen
A nitrogen-heterocyclic carbene and silylation technology, applied in the field of alcohol catalytic dehydrosilylation, can solve the problems of reduced reaction selectivity, complex synthesis process, low catalytic activity, etc., and achieve easy after-treatment, single reaction product, The effect of high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: PhSiH(OEt) 2 Synthesis
[0015] 0.0021g (0.012mmol) isopropyl azacarbene was dissolved in 0.4ml deuterated benzene (C 6 D. 6 ), add 0.051g (0.468mmol) of phenylsilane and 0.043g (0.936mmol) of ethanol successively at room temperature, immediately there are violent bubbles released, and the reaction is monitored by NMR. After 0.5 hours, the reaction ends, the conversion rate exceeds 95%, and the selectivity More than 95%.
[0016] Characterization:
[0017] 1 H NMR (C 6 D. 6 , 400MHz): δ7.65-7.75(m, 2H), 7.22–7.19(m, 3H), 5.20(s, 1H), 3.76(q, 4H), 1.13(t, 6H). 13 C NMR (C 6 D. 6 , 101MHz): δ136.13, 134.51, 130.81, 59.47, 18.48.
Embodiment 2
[0018] Example 2: PhSi(OEt) 3 Synthesis
[0019] Put 0.0084g (0.047mmol) of isopropyl azacarbene into a 10ml Shrek tube, add 0.202g (1.87mmol) of phenylsilane and 0.344g (7.48mmol) of ethanol in turn at room temperature, immediately there will be violent release of bubbles , After 1 hour, the reaction was over, no bubbles escaped, nuclear magnetic monitoring showed that the reaction was complete, the conversion rate exceeded 95%, and the product was only PhSi(OEt) 3 . The product was separated by atmospheric distillation, and the separation yield was 70%.
[0020] Characterization:
[0021] 1 H NMR (CDCl 3 , 400MHz): δ7.55-7.65(m, 2H), 7.32–7.19(m, 3H), 3.80(q, 4H), 1.16(t, 6H). 13 C NMR (CDCl 3 , 101MHz): δ134.88, 131.01, 130.42, 127.93, 58.80, 18.30.
Embodiment 3
[0022] Example 3: PhSiH(O i Pr) 2 Synthesis
[0023] Put 0.0084g (0.047mmol) of isopropyl azacarbene into a 10ml Shrek tube, add 0.202g (1.87mmol) of phenylsilane and 0.224g (3.74mmol) of isopropanol successively at room temperature, and there will be bubbles immediately After 0.5 hours, nuclear magnetic monitoring showed that the reaction was complete, and the conversion rate was 90%. The product was separated by atmospheric distillation, and the separation yield was 80%.
[0024] Characterization:
[0025] 1 H NMR (CDCl 3 , 400MHz): δ7.75-7.65(m, 2H), 7.45–7.35(m, 3H), 5.00(s, 1H), 4.27(sept, 2H), 1.16(m, 12H). 13 C NMR (CDCl 3 , 101MHz): δ135.97, 134.24, 130.51, 128.00, 66.48, 25.61.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com