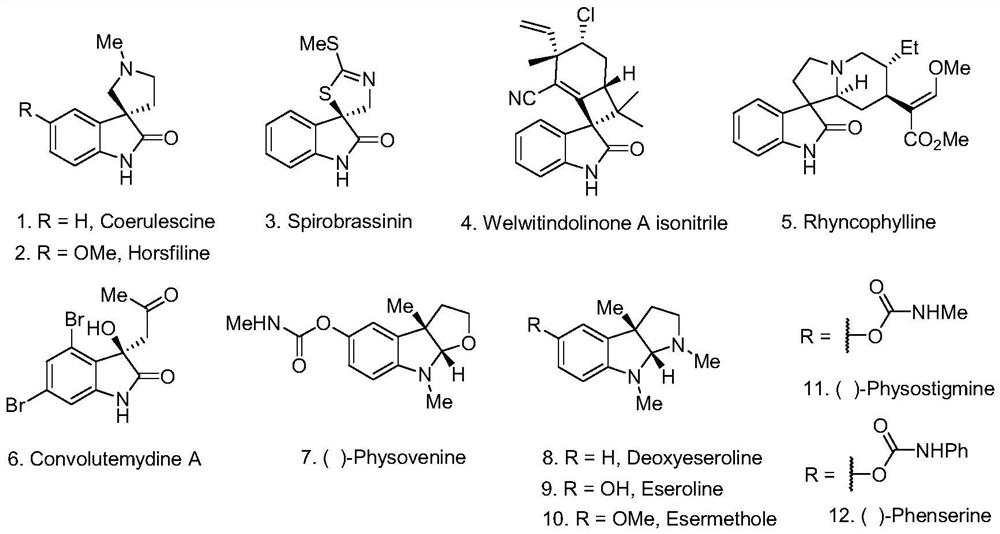
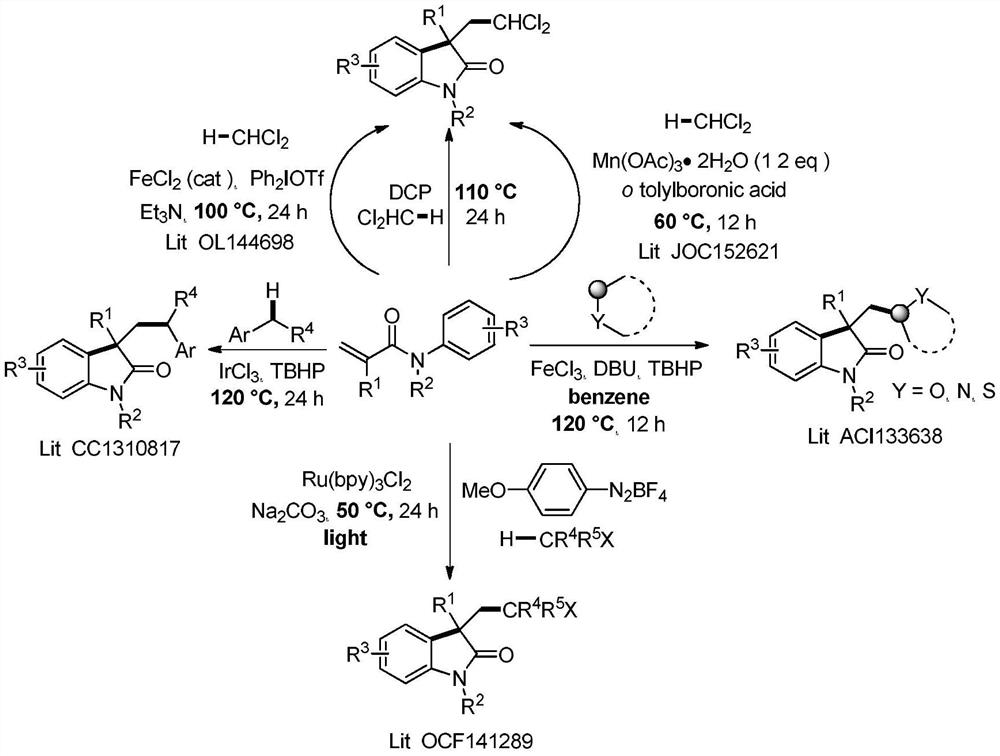
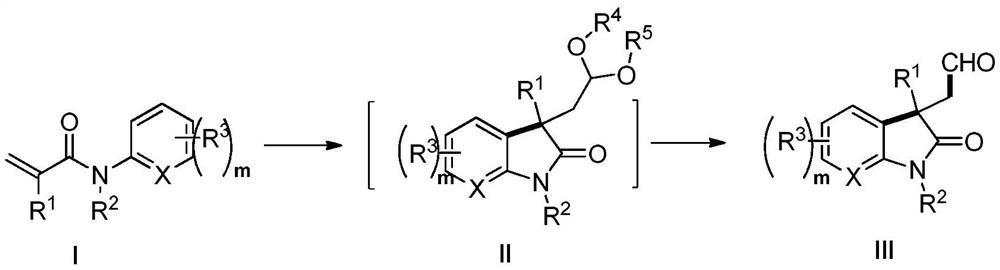
Photocatalytic method for synthesizing alkaloids
A compound and photocatalytic technology, applied in the field of photocatalytic synthesis of alkaloids, synthesis of indole fused ring compounds or indole spiro compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112]
[0113] Add N-methyl-N-phenylacrylamide (88mg, 0.5mmol) and organic cyano-carbazole photocatalyst 4CzIPN (8mg, 0.01mmol) into the photoreactor, add 1,3-di Oxypentane (3mL), tert-butanol peroxide TBHP (1.0mmol, 0.13mL), irradiated with 5W blue light and stirred at room temperature for 12 hours, monitored by TLC, the reaction of raw materials was complete, concentrated under reduced pressure, added acetone (2mL), 2M HCl Aqueous solution (2 mL), stirred at room temperature for 12 hours. After carefully quenching the reaction by addition of sodium bicarbonate solution, it was extracted with ethyl acetate (3 x 20 mL). The organic phases were combined, washed with saturated brine (20 mL), dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and purified to obtain a colorless oily substance 2-(1,3-dimethyl-2-oxoindole (olin-3-yl)acetaldehyde (83 mg, 82%). 1 H NMR (600MHz, CDCl 3 )δ1.42(s,3H,CH 3 ), 2.94 (d, J=17.2Hz, 1H, CH 2 ), 2.99 (d,...
Embodiment 2
[0115]
[0116]In the photoreactor, add methyl 4-(N-methacrylamide) benzoate (117mg, 0.5mmol), organic cyano-carbazole photocatalyst 4CzIPN (8mg, 0.01mmol), add under nitrogen atmosphere 1,3-Dioxolane (3mL) and tert-butanol peroxy TBHP (1.0mmol, 0.13mL), irradiated with 5W blue light and stirred at room temperature for 12 hours, monitored by TLC, the reaction of raw materials was complete, concentrated under reduced pressure, added acetone ( 2 mL), 1M aqueous HCl (2 mL), and stirred at room temperature for 12 hours. After carefully quenching the reaction by addition of sodium bicarbonate solution, it was extracted with ethyl acetate (3 x 20 mL). The organic phases were combined, washed with saturated brine (20 mL), dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and purified to obtain a white solid methyl 1,3-dimethyl-2-oxo-3-(2 -Oxoethyl)indoline-5-carboxylate (82 mg, 63%). 1 H NMR (600MHz, CDCl 3 )δ1.41(s,3H,CH 3 ), 3.08 (d, J=18.2H...
Embodiment 3
[0118]
[0119] 2-(1,3-Dimethyl-2-oxoindol-3-yl)acetaldehyde (102mg, 0.5mmol) was dissolved in tetrahydrofuran (5mL) solvent, and 2.4 M tetrahydrofuran solution of lithium aluminum tetrahydrogen (0.85mL, 2.0mmol, 4.0equiv.), after stirring for five minutes at 0°C, add 10mL of ethyl acetate and continue the reaction at 0°C for 30 minutes to consume the remaining lithium aluminum tetrahydride. Then add 10 mL of saturated sodium chloride solution to quench the reaction, extract with ethyl acetate (3×20 mL), combine the organic phases, wash with saturated brine (20 mL), dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure, and purify (3aS,8aS)-3a,8-dimethyl-3,3a,8,8a-tetrahydro-2H-furo[2,3-b]indole (85mg, 90%) was obtained as a colorless oil . 1 H NMR (600MHz, CDCl 3 )δ1.46(s,3H,CH 3 ),2.02-2.07(m,1H,CH 2 ), 2.12 (ddd, J=1.1, 5.2, 11.9Hz, 1H, CH 2 ),2.92(s,3H,CH 3 ), 3.45 (ddd, J=5.2, 8.7, 11.2Hz, 1H, CH 2 ),3.93-3.96(m,1H,CH 2 ), 6.36(d, J=7.8H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com