AE-active ester chemical synthesizing method
A chemical synthesis and active ester technology, applied in the direction of organic chemistry, can solve the problems of environmental pollution, ineffective use of by-products, high synthesis cost, etc., achieve no three wastes, great implementation value and social and economic benefits, and reduce production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
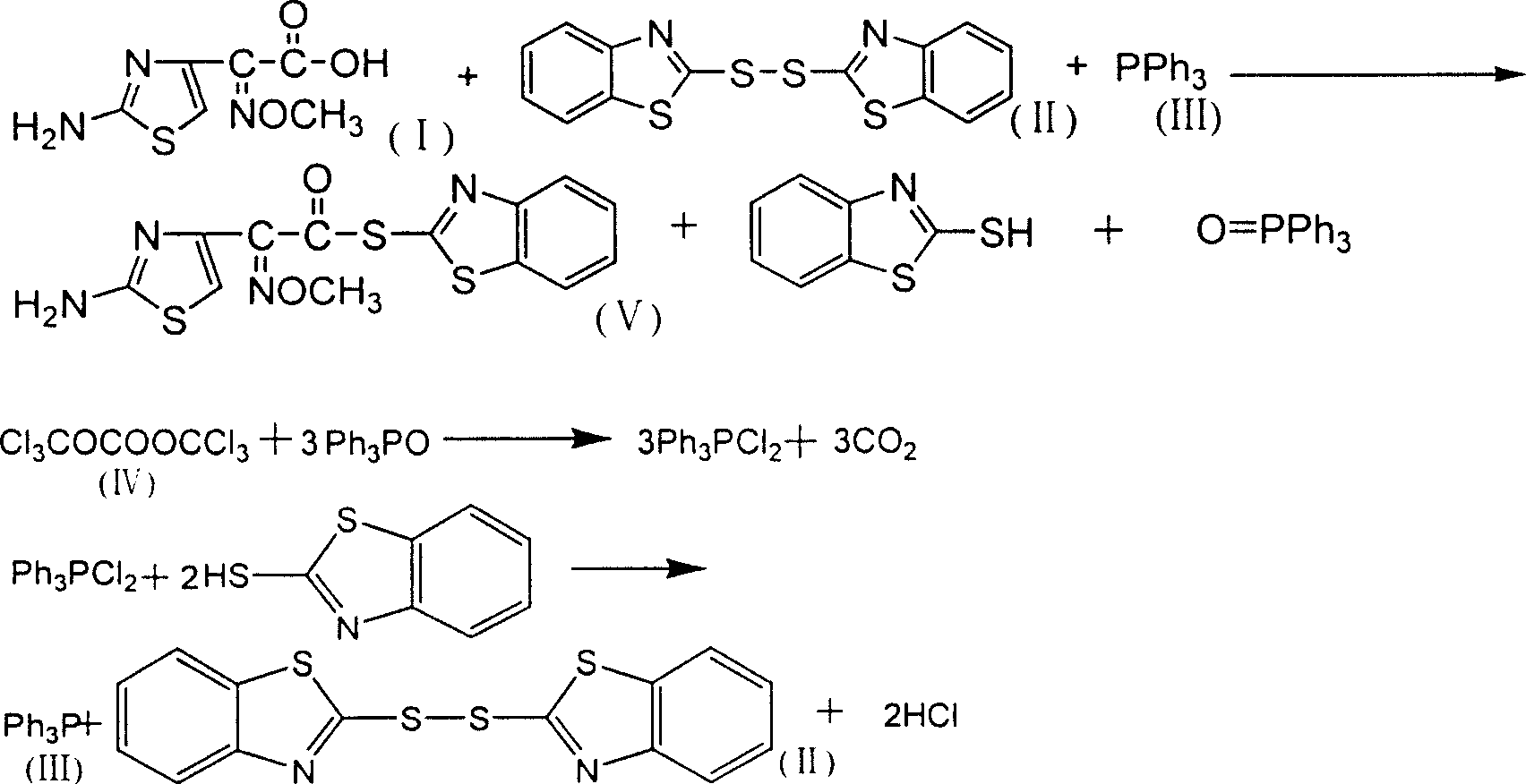
[0022] The feeding molar ratio is aminothioxime acetic acid: dibenzothiazole sulfide: triphenylphosphine: bis(trichloromethyl) carbonate: pyridine=1:1.2:1.2:0.35:0.5.
[0023] In a 500ml four-neck flask equipped with mechanical stirring, constant pressure dropping funnel, reflux condenser and thermometer, add 20g (0.0995mol) of aminothioxime acetic acid, add dibenzothiazole sulfide, pyridine and 100ml of dichloro Methane, start stirring, slowly add triphenylphosphine 80ml dichloromethane solution dropwise under normal temperature and vigorous stirring, after the addition is complete, stir and react at 20-25°C for 3h (t1), cool in an ice bath, filter, The filter cake was washed with methanol and dried under vacuum to obtain 29.1 g (0.08314 mol, theoretical value 0.0995 mol) of AE-active ester, with a yield of 83.6% and a content of 98.6% (LC); (Trichloromethyl)carbonate in 50ml of dichloromethane solution, after the addition, continue to react at 20-25°C for 3 hours (t2), after...
Embodiment 2
[0025] The feeding molar ratio is aminothioxime acetic acid: dibenzothiazole sulfide: triphenylphosphine: bis(trichloromethyl) carbonate: pyridine=1:1.2:1.2:0.5:1.0.
[0026] In a 500ml four-neck flask equipped with mechanical stirring, a constant pressure dropping funnel, a reflux condenser and a thermometer, add 20g of aminothioxime acetic acid, add dibenzothiazole sulfide (partially recovered, partly newly added), pyridine and 80ml of dichloromethane, start stirring, and slowly dropwise add 70ml of dichloromethane solution of triphenylphosphine (partially recovered, partly newly added) at normal temperature and under vigorous stirring, after the addition is completed, the temperature is raised, and at 25-30°C Stir the reaction at high temperature for 4h (t1), cool in an ice bath, filter, wash the filter cake with methanol, and dry in vacuo to obtain 29.8g (0.085mol) of AE-active ester, with a yield of 85.4% and a content of 98.7% (LC); The filtrate was slowly added dropwise...
Embodiment 3
[0028] The molar ratio of feeding is aminothioxime acetic acid: dibenzothiazole sulfide: triphenylphosphine: bis(trichloromethyl) carbonate: pyridine=1:1.2:1.2:1.5:1.0.
[0029] In a 500ml four-neck flask equipped with mechanical stirring, a constant pressure dropping funnel, a reflux condenser and a thermometer, add 20g of aminothioxime acetic acid, add dibenzothiazole sulfide (partially recovered, partly newly added), pyridine and 100ml of dichloromethane, start stirring, slowly dropwise add 80ml of dichloromethane solution of triphenylphosphine (partially recovered, partly newly added) at normal temperature and under vigorous stirring, after adding, heat up, and The reaction was stirred at high temperature for 4h (t1), cooled in an ice bath, filtered, the filter cake was washed with methanol, and dried in vacuo to obtain 28.7g (0.082mol) of AE-active ester with a yield of 82.4% and a content of 98.1% (LC); The filtrate was slowly added dropwise with 100ml of dichloromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com