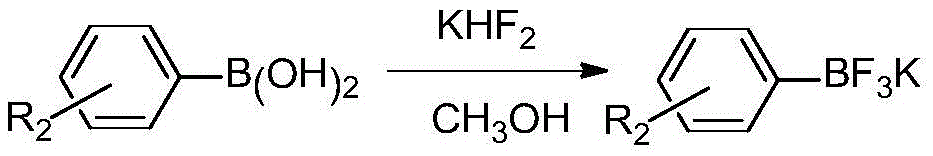
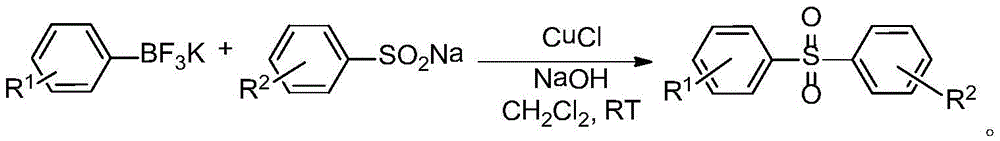
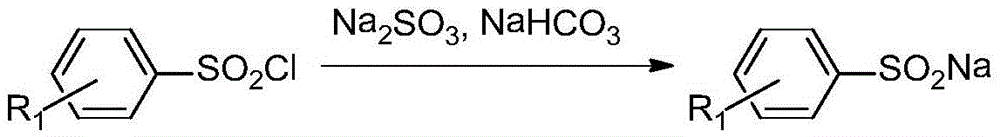Asymmetric diaryl sulfone compound and its preparation method
An asymmetric, diaryl technology, applied in the field of diaryl sulfone and its preparation, can solve problems such as not having practical value, and achieve the effects of high practicability and selectivity, mild conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 1 mmol of sodium p-methoxybenzenesulfinate, 1 mmol of potassium phenylfluoroborate, 1.2 mmol of sodium hydroxide and 0.1 mmol of cuprous chloride into a reaction tube containing 2 ml of dichloromethane, and stir magnetically for 3 hours. After the reaction is completed, filter through filter paper, spin dry the solvent, and separate by column to obtain the product. The yield was 95%.
[0035] 1-(4-Methoxyphenylsulfonyl)benzene Yellow solid(mp=90-92℃,lit mp88-90℃); 1 H NMR (300MHz, CDCl3) δ7.90 (m, 4H), 7.51 (m, 3H), 6.96 (m, 2H), 3.84 (s, 3H); HRMS calcd forC 13 h 12 o 3 S:248.0507,found:248.0509.
Embodiment 2
[0037] Add 1 mmol of sodium p-nitrobenzenesulfinate, 1 mmol of potassium phenylfluoroborate, 1.2 mmol of sodium hydroxide and 0.1 mmol of cuprous chloride into a reaction tube containing 2 ml of dichloromethane, and stir magnetically for 3 hours. After the reaction is completed, filter through filter paper, spin dry the solvent, and separate by column to obtain the product. The yield was 96%.
[0038] 1-(4-Nitrophenylsulfonyl)benzene Yellow solid(mp=145-149℃,lit mp143-145℃); 1 H NMR (400MHz, CDCl 3 ):δ8.27–8.38(m,2H),8.07–8.15(m,2H),7.92–8.00(m,2H),7.58–7.66(m,1H),7.51–7.61(m,2H).HRMS calcd for C 12 h 9 NO 4 S:263.0252,found:263.0250.
Embodiment 3
[0040] Add 1mmol of sodium benzenesulfinate, 1mmol of potassium p-bromophenylfluoroborate, 1.2mmol of sodium hydroxide and 0.1mmol of cuprous chloride into a reaction tube containing 2ml of dichloromethane, and stir magnetically for 3 hours. After the reaction is completed, filter through filter paper, spin dry the solvent, and separate by column to obtain the product. The yield was 94%.
[0041] 1-Bromo-4-(phenylsulfonyl)benzene Yellow solid (mp=100-101℃, lit mp98-99℃); 1 H NMR (400MHz, CDCl 3 ):δ7.88–7.99(m,2H),7.76–7.86(m,2H),7.61–7.69(m,2H),7.55–7.61(m,1H),7.46–7.52(m,2H).HRMS calcd for C 12 h 9 BrO 2 S:295.9507,found:295.9508.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com