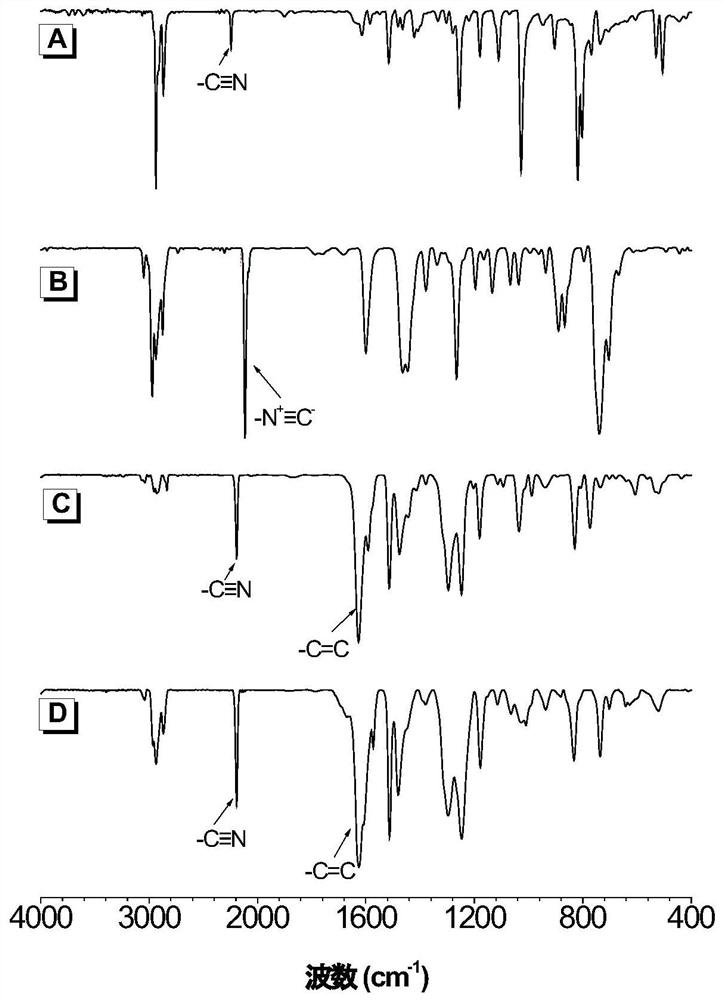
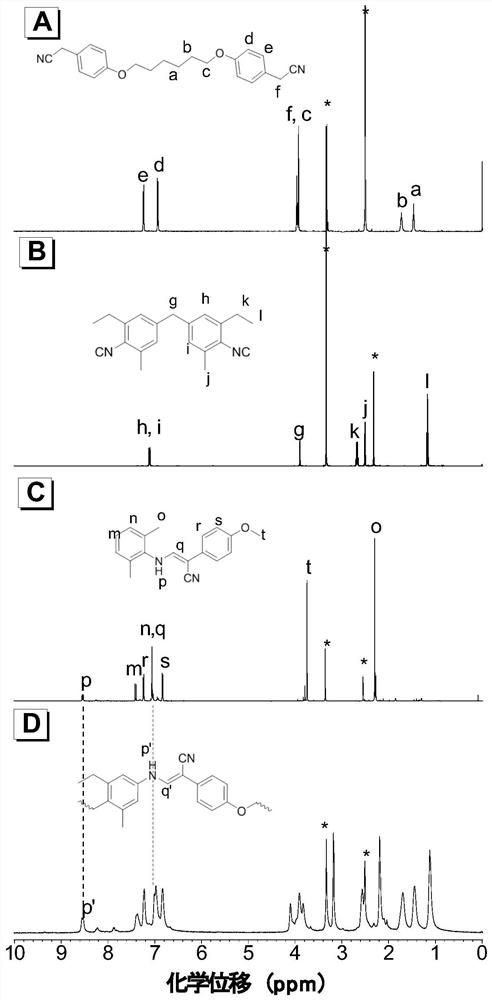
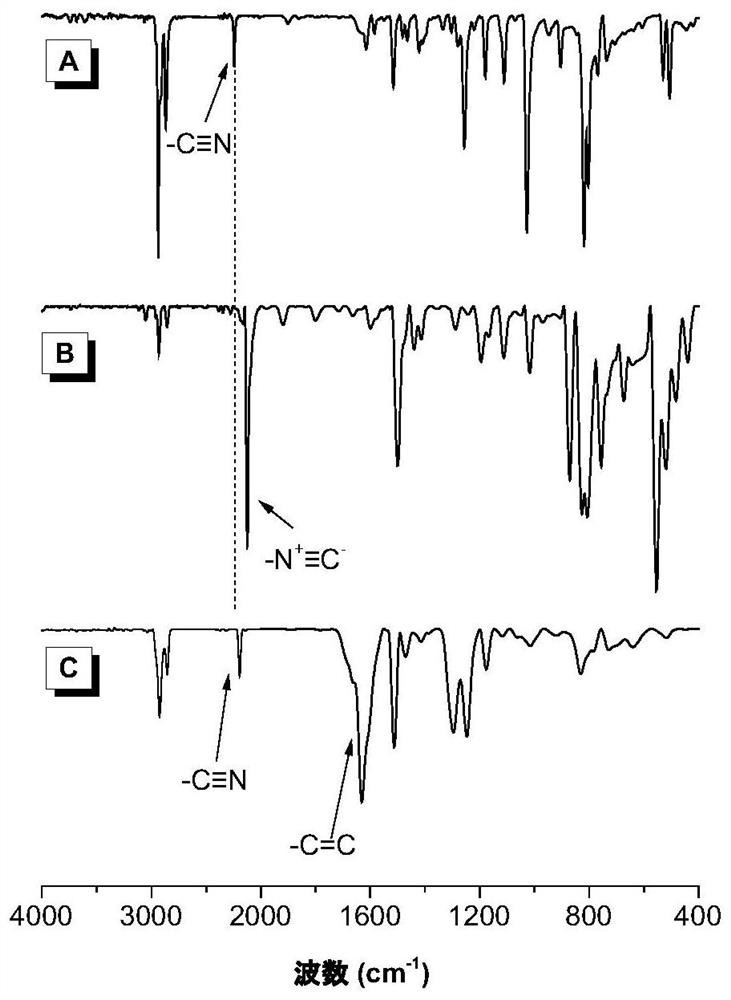
Poly(enamine nitrile) compound and preparation method thereof
A technology of polyenaminonitrile and compound, which is applied in the field of polyenaminonitrile compounds and their preparation, can solve the problems of blank polymers and their synthesis methods, and achieve high polymerization efficiency, easy availability of reaction raw materials, and high thermal stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] (1) The synthetic method of the first monomer binary isocyanide compound 1a is as follows,
[0060]
[0061] (2) The synthetic method of the second monomer binary nitrile compound 2a is as follows,
[0062]
[0063] (3) Preparation of polyenylamine nitrile compounds P1a2a by nitrile-isonitrile polymerization
[0064]
[0065] Add 69.5mg (0.20mmol) of the first monomer 1a, 60.5mg (0.20mmol) of the second monomer 2a, 3.8mg (0.02mmol) of cuprous iodide and 53.9mg (0.48mmol) of potassium tert-butoxide into a 10mL polymerization tube ), replace nitrogen three times, add 2mL of N,N-dimethylformamide, after the monomer is completely dissolved, raise the temperature to 40°C. React for 4 hours. The reaction solution was diluted with 2 mL of N,N-dimethylformamide, and added dropwise into 150 mL of rapidly stirring methanol through a cotton filter to obtain a white flocculent precipitate. Stand overnight, filter and dry to obtain the target polymer.
[0066] The polym...
Embodiment 2~3
[0073] Examples 2-7 investigated the influence of the reaction solvent on this polymerization reaction. The preparation of the polymer monomer was the same as in Example 1. The reaction conditions and results of step (3) were shown in Table 1.
[0074] The effect of table 1 reaction solvent on monomer 1a and 2a polymerization a
[0075]
[0076] a Reaction in different solvents under nitrogen for 4h; T=40°C; [M] 0 = 100 mM.
[0077] b Determined by GPC, using linear polymethyl methacrylate as a calibration substance and DMF as a mobile phase.
[0078] By comparing the data in Table 1, we found that the polymerization reaction can only occur in polar solvents N,N-dimethylformamide and dimethyl sulfoxide, because the prepared polyenamide can only be dissolved in polar solvents , poor solubility in solvents such as tetrahydrofuran, which limits the chain growth of the polymer. Compared with the polymerization results of N,N-dimethylformamide and dimethyl sulfoxide as poly...
Embodiment 8~14
[0080] Examples 8-14 investigated the influence of different temperatures on the reaction conditions. The preparation of polymerized monomers is the same as in Example 1. The reaction conditions and results of step (3) are shown in Table 2.
[0081] Table 2 Effect of Temperature on the Polymerization of Monomers 1a and 2a a
[0082]
[0083] a Reaction in N,N-dimethylformamide for 4h in nitrogen; [M] 0 = 100 mM.
[0084] b Determined by GPC, using linear polymethyl methacrylate as a calibration substance and DMF as a mobile phase.
[0085] As can be seen from Table 2, at room temperature (25 ° C), the polymerization reaction can also occur, and the yield is higher (70%), and the molecular weight is larger (M w =11200), which shows that the polymerization reaction can be economical, energy-saving and environmentally friendly at room temperature. When the reaction temperature is below 40°C, the higher the temperature, the higher the polymerization yield and the larger t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com