Tetrahydrobenzofuran Mannich alkali compounds, and preparation method and application thereof
A technology for benzofuran and compounds, which is applied in the field of tetrahydrobenzofuran Mannich base compounds, can solve environmental pollution and other problems, and achieve the effects of simple post-processing, low environmental pollution, and good functional group tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of Tetrahydrobenzofuranocyclohexanone Mannich Base
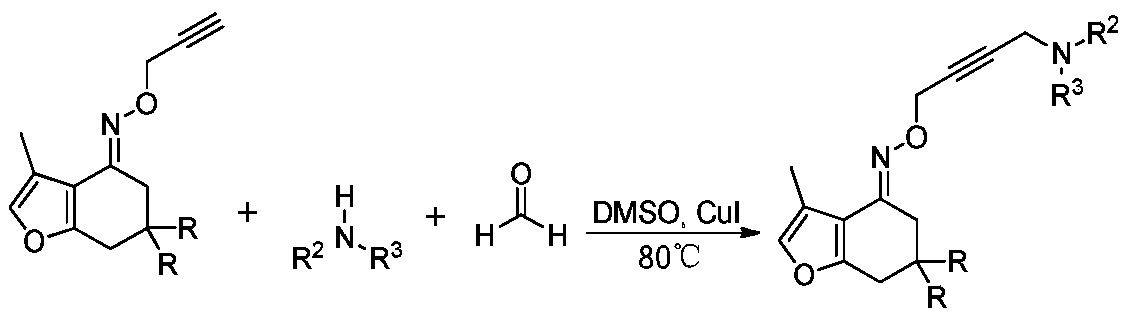
[0022] Using tetrahydrobenzofuran-4-ketoxime ether as a raw material, the reaction route for the synthesis of long-chain Mannich bases by reacting with secondary amines and formaldehyde on terminal alkynes is as follows:
[0023]
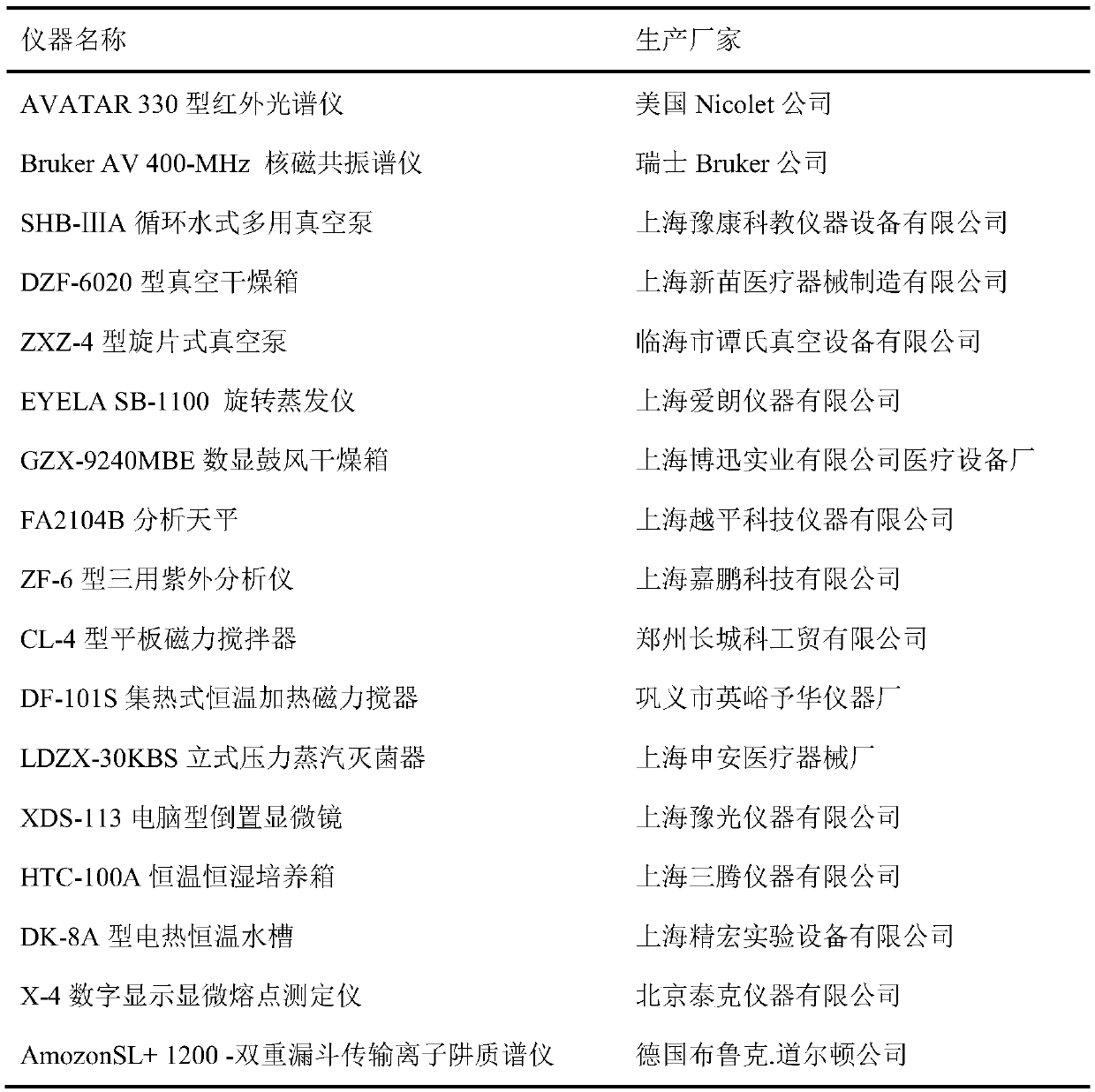
[0024] Table 1 Experimental Instruments
[0025]
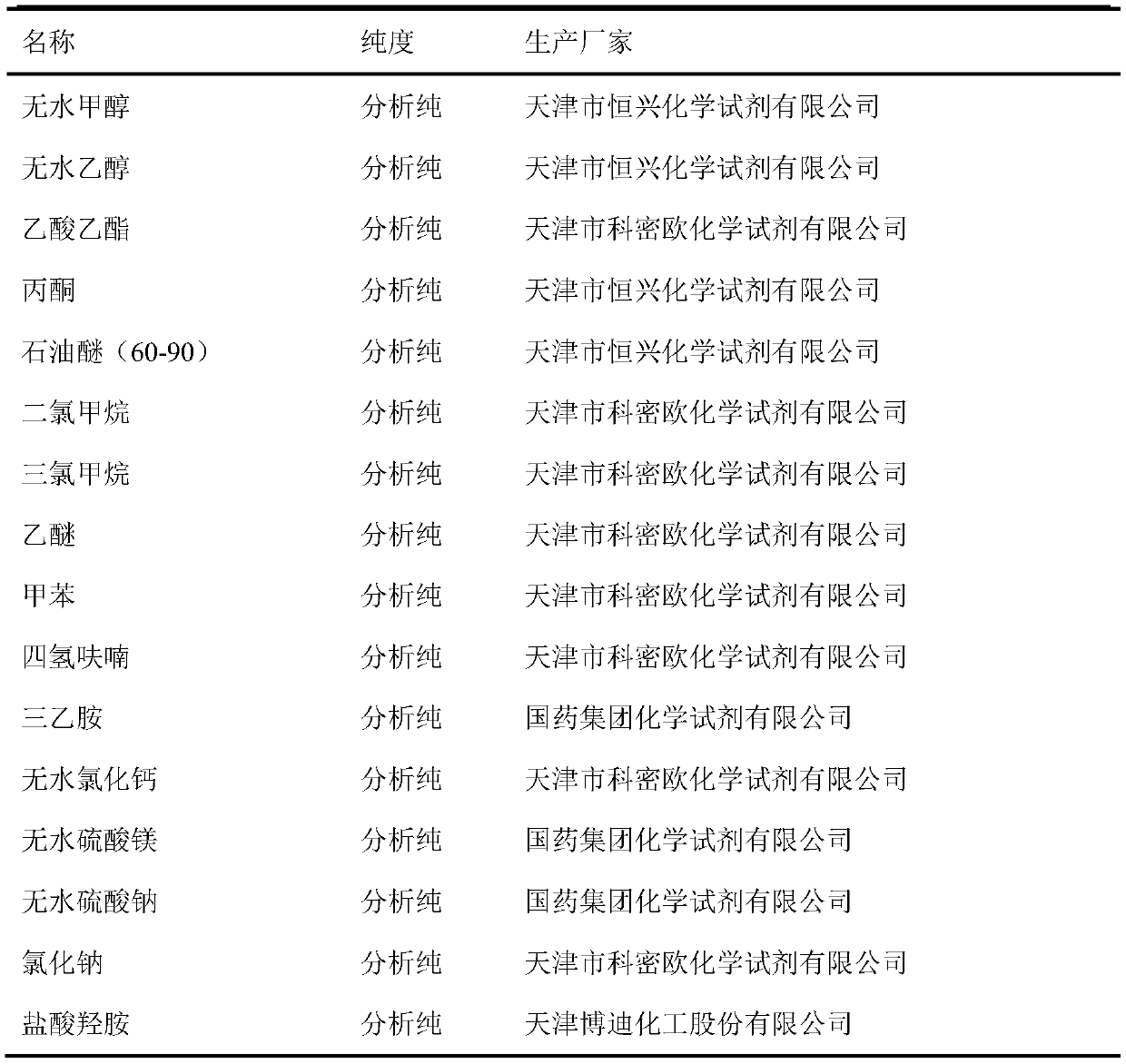
[0026] experimental reagent
[0027] Table 2 Experimental Reagents
[0028]
[0029] The reagents used were all domestic and commercially available analytically pure, and the column chromatography silica gel (200-300 mesh) was produced by Qingdao Ocean Chemical Co., Ltd. All reagents in the reaction were pretreated according to the "Reagent Purification Manual" before use.
[0030] Synthesis of intermediates
[0031]
[0032] Dissolve 3-methyl-6,7-dihydrobenzofuran-4(5H)-one (compound 1a, 1.78 g, 10.0 mmol) and hydroxylamine hydrochloride (0.834 g, 12.0 mmol) in 30 mL of anhydrous methanol, and then Anhydrous sod...
Embodiment 2
[0089] Preparation of Heterocyclic Mannich Base 5
[0090]
[0091] Put intermediate 3b (1.0mmol), p-chloroaniline (1.5mmol) and 30% aqueous formaldehyde (4.0mmol) into a 50mL round bottom flask, add 10mL ethylene glycol to dissolve and catalytic amount of glacial acetic acid, heat to 80°C, Stir at constant temperature for a certain time, stir, TLC monitors until the reaction is complete, pour the reaction solution into 30mL water, extract with ethyl acetate (3×20mL), dry, and distill the organic phase to remove the solvent under reduced pressure, and the obtained crude product is purified by column chromatography ( Petroleum ether: ethyl acetate = 10:1) to obtain the heterocyclic Mannich base 5a.
[0092] (E)-2-(((4-Chlorophenyl)amino)methyl)-3,6,6-trimethyl-6,7-dihydrobenzofuran-4(5H)-one O-prop-2-yn-1-yl oxime (5a): Yellow oil, yields 61%; R f 0.45(ethyl acetate / petroleum ether=1:30,v / v); 1 H NMR (CDCl 3 ,400MHz)δ(ppm):7.14-7.11(m,2H),6.61-6.57(m,2H),4.68(d,J=2.4Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com