Polycyclic carbamoyl pyridone analogue and preparation method and application thereof
A technology of polycyclic carbamoyl pyridone and cyclic carbamoyl pyridone, which is applied in the field of medicinal chemistry, can solve the problems of poor patient compliance, slow onset time and the like, achieves good functional group tolerance, the method is simple to operate, and the process Economical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
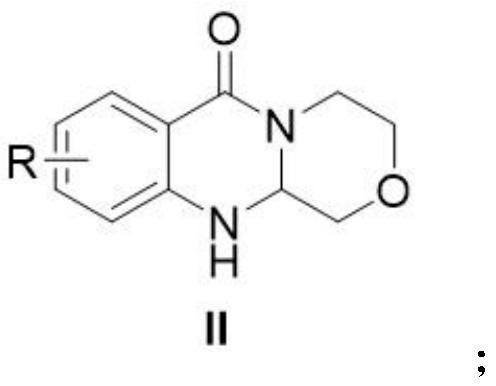
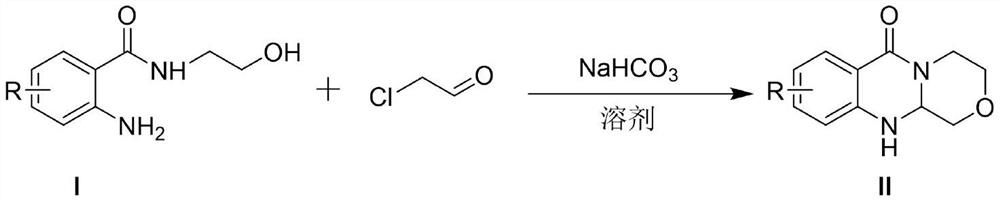
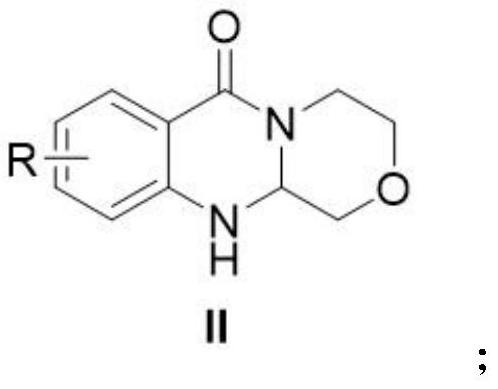
[0033] Add the compound of formula I, chloroacetaldehyde and 1,4-dioxane into a dry and clean reaction flask, stir and raise the temperature to 100°C for 5 hours, then add sodium bicarbonate to continue the reaction for 3 hours, and monitor the reaction by TLC. Cool to room temperature, add water / ethyl acetate to extract 3 times, combine the organic phases, add saturated brine to wash, collect the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure, the residue is purified to obtain the target product.
[0034] In an initial experiment, the inventors performed the cyclization of 2-amino-N-(2-hydroxyethyl)benzamide with chloroacetaldehyde to synthesize 3,4,11,11a-tetrahydro-[1,4]oxazine The reaction conditions of [3,4-b]quinazolin-6(1H)-one were explored, and the results are listed in Table 1. The first reaction condition tried was NaHCO 3 (1.2 equivalents) as a catalyst, in 1,4-dioxane as a solvent at 100 ° C for 8 hours. Reassur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com