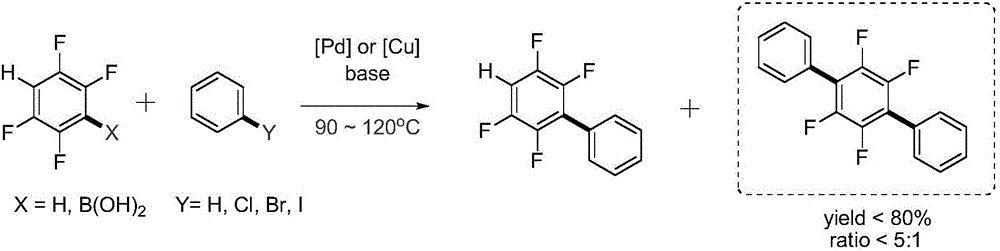
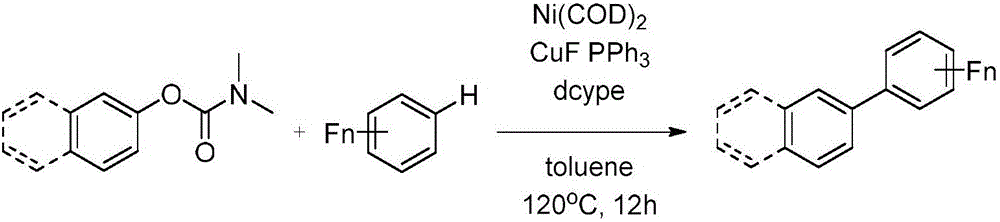
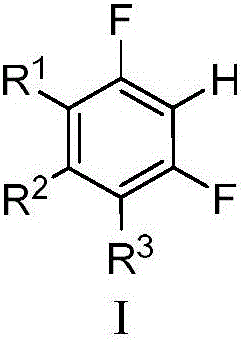
Magnesium assisted nickel catalyzed multi-fluoro aromatic hydrocarbon monoarylation method
A technology of polyfluoroaromatics and nickel catalysis, which is applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve problems such as difficult to achieve high-efficiency monoarylation of polyfluoroaromatics and harsh reaction conditions, and achieve Effects of high industrial production prospects, easy post-processing, high economic value and social value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of 2-methyl-5-(2′,3′,5′,6′-tetrafluorophenyl)thiophene with the following structural formula
[0044]
[0045] 1. Under anhydrous and oxygen-free conditions, dissolve 360mg (2.4mmol) of 1,2,4,5-tetrafluorobenzene in 1mL of tetrahydrofuran, then add 1mL of 2mol / L tetrahydrofuran solution of isopropylmagnesium chloride, and stir the reaction at room temperature After 12 hours, 3 mL of 1,4-dioxane, 0.6 mL of ethylene glycol dimethyl ether and 26.4 mg (0.10 mmol) of 18-crown-6 were added to the reaction solution, and stirring was continued at room temperature for 30 minutes.
[0046] 2. Under anhydrous and oxygen-free conditions, add 20.6g (0.08mmol) bis(1,5-cyclooctadiene) nickel, 77mg (0.1mmol ) Bis(2-bis(3,5-dimethyl-4-methoxyphenyl)phosphine)phenyl ether, stirred at room temperature for 30 minutes.
[0047] 3. Under anhydrous and oxygen-free conditions, mix the reaction solution obtained in step 1 and step 2, and add 224mg (1.0mmol) 2-methyl-5-iodothiophen...
Embodiment 2
[0049] Synthesis of 4-(2′,3′,5′,6′-tetrafluorophenyl)acetophenone with the following structural formula
[0050]
[0051] In step 2 of Example 1, bis(2-bis(3,5-dimethyl-4-methoxyphenyl)phosphine) phenyl ether was mixed with equimolar bis(2-bis(2-naphthyl) ) phosphine) phenyl ether replacement, in step 3, 2-methyl-5-iodothiophene is replaced with equimolar p-acetylphenyl trifluoromethanesulfonate, the reaction temperature is reduced to 10 ° C, other steps and implementation Same as Example 1, 4-(2′,3′,5′,6′-tetrafluorophenyl)acetophenone was obtained, the yield was 85%, and the structural characterization data was: 1 H NMR (400MHz, CDCl 3 ): δ8.08 (d, J = 8.4Hz, 2H), 7.58 (d, J = 8.0Hz, 2H), 7.15-7.10 (m, 1H), 2.66 (s, 3H).
Embodiment 3
[0053] Synthesis of 2,3,5,6-tetrafluoro-1,1'-biphenyl with the following structural formula
[0054]
[0055] In step 3 of Example 1, 2-methyl-5-iodothiophene was replaced with equimolar phenyl triflate, and the reaction temperature was raised to 40°C. Other steps were the same as in Example 1 to obtain 2 ,3,5,6-Tetrafluoro-1,1′-biphenyl, the yield is 91%, and the structural characterization data are: 1 H NMR (400MHz, CDCl 3 ):δ7.54-7.44(m,5H),7.13-7.01(m,1H);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com