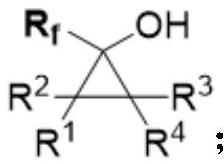
Alpha-fluoroalkyl substituted cyclopropyl alcohol compound as well as preparation method and application thereof
A fluoroalkyl and cyclopropyl technology, applied in the field of organic synthesis, can solve the problem of uncontrollable substrate regioselectivity, and achieve good substrate universality, good functional group tolerance, and good diastereoselectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
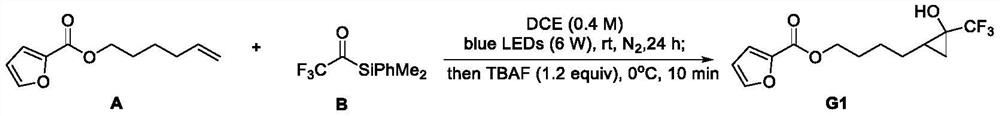
[0033] In a glove box, A (38.8 mg, 0.2 mmol), dried DCE (0.5 mL) and B (92.8 mg, 0.4 mmol, 2 equiv.) were added to a dry photoreaction tube equipped with a magnetic stir bar. The photoreaction tube was sealed, taken out from the glove box, and then irradiated with a 6W blue LED lamp, and the reaction mixture was stirred at room temperature for 24 hours. Then the blue light was turned off, TBAF (0.24 mL, 1.0 M in THF, 1.2 equiv.) was added, and the mixture was stirred under ice bath for 10 min. The reaction mixture was concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (200-300 mesh), and eluted with PE / EA (20 / 1-10 / 1, v / v) to obtain the target compound G1 (52.6 mg of colorless oily liquid, yield 90% ). 1 H NMR (400MHz, CDCl 3 ,25℃)δ7.57(dd,J=1.8,0.9Hz,1H),7.18(dd,J=3.5,0.9Hz,1H),6.51(dd,J=3.5,1.7Hz,1H),4.49– 4.21(m,2H),2.97(s,1H),1.88–1.77(m,2H),1.61–1.48(m,4H),1.26–1.15(m,2H),0.69–0.58(m,1H). 13 C NMR (151MH...
Embodiment 2
[0035]
[0036] In a glove box, A (20.8 mg, 0.2 mmol), dry DCM (0.5 mL) and B (92.8 mg, 0.4 mmol, 2 equiv.) were added to a dry photoreaction tube equipped with a magnetic stir bar. The photoreaction tube was sealed, taken out from the glove box, and then irradiated with a 6W blue LED lamp, and the reaction mixture was stirred at room temperature for 24 hours. Then the blue light was turned off, TBAF (0.24 mL, 1.0 M in THF, 1.2 equiv.) was added, and the mixture was stirred under ice bath for 10 min. The reaction mixture was concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (200-300 mesh), and eluted with PE / EA (50 / 1-10 / 1, v / v) to obtain the target compound G2 (colorless oily liquid 28.3mg, yield 70% ). 1 H NMR (600MHz, CDCl 3 , 25°C) δ7.38-7.31(m, 2H), 7.31-7.27(m, 1H), 7.25-7.21(m, 2H), 2.58(dd, J=10.4, 7.8Hz, 1H), 2.46(s , 1H), 1.57(dd, J=10.5, 6.9Hz, 1H), 1.48-1.39(m, 1H). 19 F NMR (375MHz, CDCl 3 , 25°C) δ77.1...
Embodiment 3
[0038]
[0039] In a glove box, A (20.8 mg, 0.2 mmol), dry DCM (0.5 mL) and B (92.8 mg, 0.4 mmol, 2 equiv.) were added to a dry photoreaction tube equipped with a magnetic stir bar. The photoreaction tube was sealed, taken out from the glove box, and then irradiated with a 6W white LED lamp, and the reaction mixture was stirred at room temperature for 24 hours. Then the blue light was turned off, TBAF (0.24 mL, 1.0 M in THF, 1.2 equiv.) was added, and the mixture was stirred under ice bath for 10 min. The reaction mixture was concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (200-300 mesh), and eluted with PE / EA (50 / 1-10 / 1, v / v) to obtain the target compound G3 (colorless oily liquid 27.2mg, yield 67% ). 1 H NMR (600MHz, CDCl 3 , 25°C) δ7.38-7.31(m, 2H), 7.31-7.27(m, 1H), 7.25-7.21(m, 2H), 2.58(dd, J=10.4, 7.8Hz, 1H), 2.46(s , 1H), 1.57(dd, J=10.5, 6.9Hz, 1H), 1.48-1.39(m, 1H). 19 F NMR (375MHz, CDCl 3 , 25°C) δ77....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com