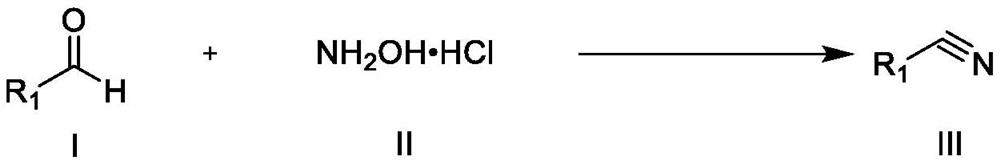Synthesis method of nitrile compound
A synthesis method and compound technology, applied in the field of biomedicine, can solve the problems of medium yield, unsuitable for large-scale industrial production, etc., and achieve the effects of good functional group tolerance and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
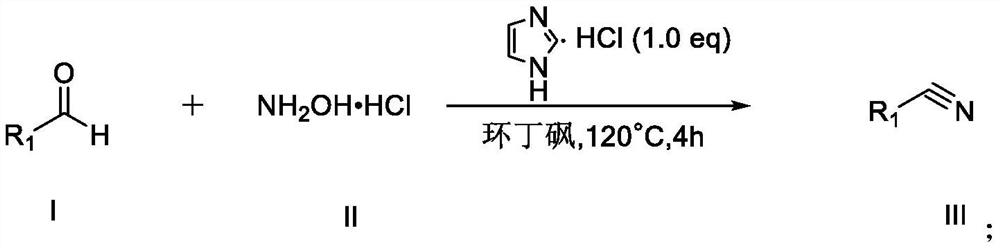
[0045] The general preparation steps that the present invention obtains are:
[0046] Add formula I compound, formula II compound and imidazole hydrochloride in 10ml round bottom flask, place 120 ℃ of oil baths and heat reaction, monitor reaction progress by TLC, after reaction finishes, add 25ml saturated common salt water and make reaction cooling, and The resulting mixture was extracted four times with 20 ml of ethyl acetate, and the combined organic layers were successively washed with anhydrous Na 2 SO 4 After drying, filtration and concentration under reduced pressure, the residue was purified by silica gel column chromatography (eluent: petroleum ether and ethyl acetate in a volume ratio of 6:1), and recrystallized from ethyl acetate and n-hexane to obtain the target product.
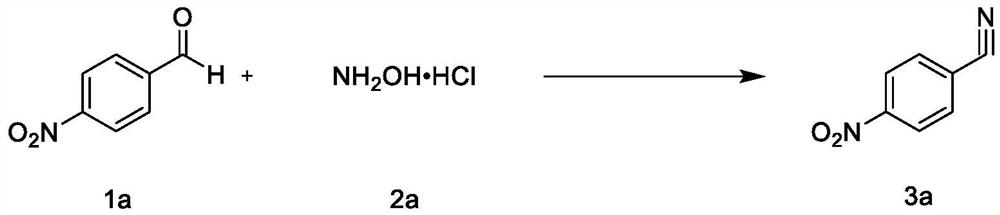
[0047]The inventor explored the reaction of 4-nitro-benzaldehyde (1a) and hydroxylamine hydrochloride (2a) to synthesize 4-nitrobenzonitrile (3a), and the results are listed in Table 1. The ini...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com