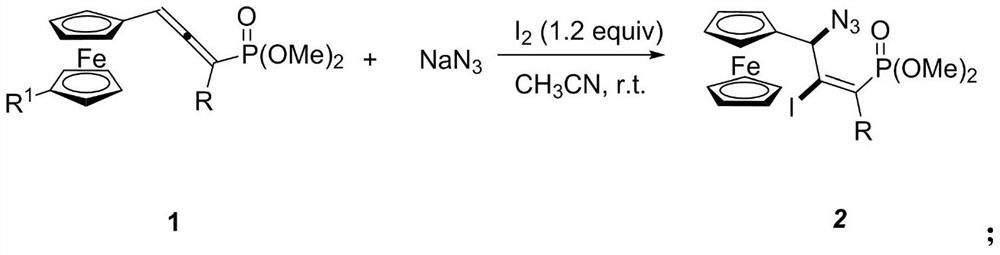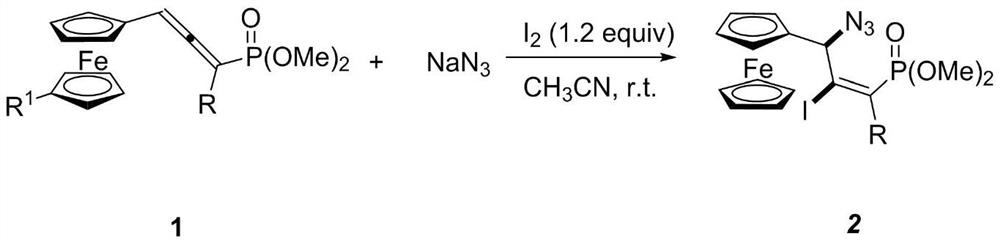Stereoselective synthesis method of tetra-substituted allyl azide
An allyl azide and stereoselective technology, applied in chemical instruments and methods, organic chemistry, metallocene, etc., can solve the problems of limited universality of substrates, cumbersome reaction steps, and low selectivity, achieving Easy operation, high yield and selectivity, fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
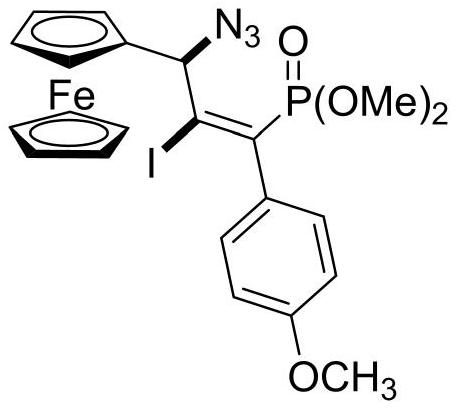
[0025] Synthesis of (E)-dimethoxy(3-ferrocenyl-3-azido-2-iodo-1-(4-methoxy)phenylpropenyl)phosphate
[0026] In the 25mL reaction flask, add ferrocene allenyl phosphate dimethyl ester compound (88 mg, 0.20mmol), NaN 3 (26mg, 0.40mmol), elemental iodine (61mg, 0.24 mmol). Then add 2.0ml CH 3 CN, stirred at room temperature and detected by TLC that the reaction was complete, separated by column chromatography, using petroleum ether: ethyl acetate (V:V) = 3:1 as the eluent, purified to obtain a yellow solid with a yield of 84%. Its NMR data are as follows:
[0027]
[0028] The NMR characterization data of the synthesized new compound: 1 H NMR(500MHz,DMSO)δ6.93(d,J=21.0Hz,4H),6.91(s,1H),4.40(s,1H),4.35(s,1H),4.26(s,5H),4.22 (s,2H),3.77(s,3H),3.74–3.58(m,6H). 31 P NMR (202MHz, DMSO) δ10.6. 13 C{ 1 H}NMR (125 MHz, DMSO) δ159.4, 140.5, 139.2, 135.5, 135.4, 134.9 (d, J pc =6.9Hz), 130.3(d,J pc =68.0Hz), 114.3, 87.6, 69.5, 69.0, 67.8 (d, J pc =13.7Hz), 67.5,65.3(d,J pc =4...
Embodiment 2
[0030] Synthesis of (E)-dimethoxy(3-ferrocenyl-3-azido-2-iodo-1-phenylpropenyl)phosphate
[0031] Add ferrocene allenyl phosphate dimethyl ester compound (81 mg, 0.20mmol), NaN 3 (26mg, 0.40mmol), elemental iodine (61mg, 0.24 mmol). Then add 2.0ml CH 3 CN, stirred at room temperature and detected by TLC that the reaction was complete, separated by column chromatography, using petroleum ether: ethyl acetate (V:V) = 3:1 as the eluent, purified to obtain a yellow solid with a yield of 72%. Its NMR data are as follows:
[0032]
[0033] 1 H NMR (500MHz, CDCl 3 )δ7.36(s,3H),7.14(s,1H),7.02(s,1H), 6.78(d,J=2.3Hz,1H),4.49(s,1H),4.42(d,J=0.8 Hz,1H),4.30(s,5H),4.23–4.18(m,2H),3.79(d,J=11.3Hz,3H),3.70(d,J=11.2Hz, 3H). 31 P NMR (202MHz, CDCl 3 )δ10.0. 13 C{ 1 H}NMR (125MHz, CDCl 3 )δ142.6(d,J pc =7.0Hz), 140.2, 138.9, 135.5, 135.3, 128.44, 128.3 (d, J pc =1.9Hz), 87.5, 69.3, 68.6, 67.7 (d, J pc =13.0Hz), 67.4, 65.4 (d, J pc =5.2Hz), 53.1(dd, J pc =7.5,6.3Hz).
Embodiment 3
[0035] Synthesis of (E)-dimethoxy(3-ferrocenyl-3-azido-2-iodo-1-methylpropenyl)phosphate
[0036] Add alkyl allenyl phosphate compound (69.2mg, 0.20mmol), NaN 3 (26mg, 0.40mmol), elemental iodine (61mg, 0.24mmol). Then add 2.0ml CH 3 CN, stirred at room temperature and detected by TLC that the reaction was complete, separated by column chromatography, using petroleum ether: ethyl acetate (V:V) = 3:1 as the eluent, purified to obtain a yellow solid with a yield of 62%. Its NMR data are as follows:
[0037]
[0038] 1 H NMR (500MHz, DMSO) δ6.79(s,1H),4.35(s,1H),4.22(s,5H),4.20(s,2H),4.17(s,1H),3.77(t,J= 12.4Hz,6H),2.02(d,J=11.9Hz,3H). 31 P NMR (202MHz, DMSO) δ13.9. 13 C{ 1 H}NMR(125MHz, DMSO)δ132.8(dd,J pc =88.9,71.1Hz),87.8,69.8,69.4,68.9,67.7(d, J pc =18.4Hz), 67.4, 65.6(d, J pc =5.8Hz), 53.2(d, J pc =5.0Hz), 28.4(d, J pc= 8.9Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com