Method for preparing aliphatic polyesters
A technology of aliphatic polyester and caprolactone, which is applied in the field of biodegradable polymer synthesis, can solve the problems of difficult recycling, difficult to control the properties of aliphatic polyester, and expensive catalysts, etc., and achieve a narrow molecular weight distribution index , high biological safety, the effect of less catalyst consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
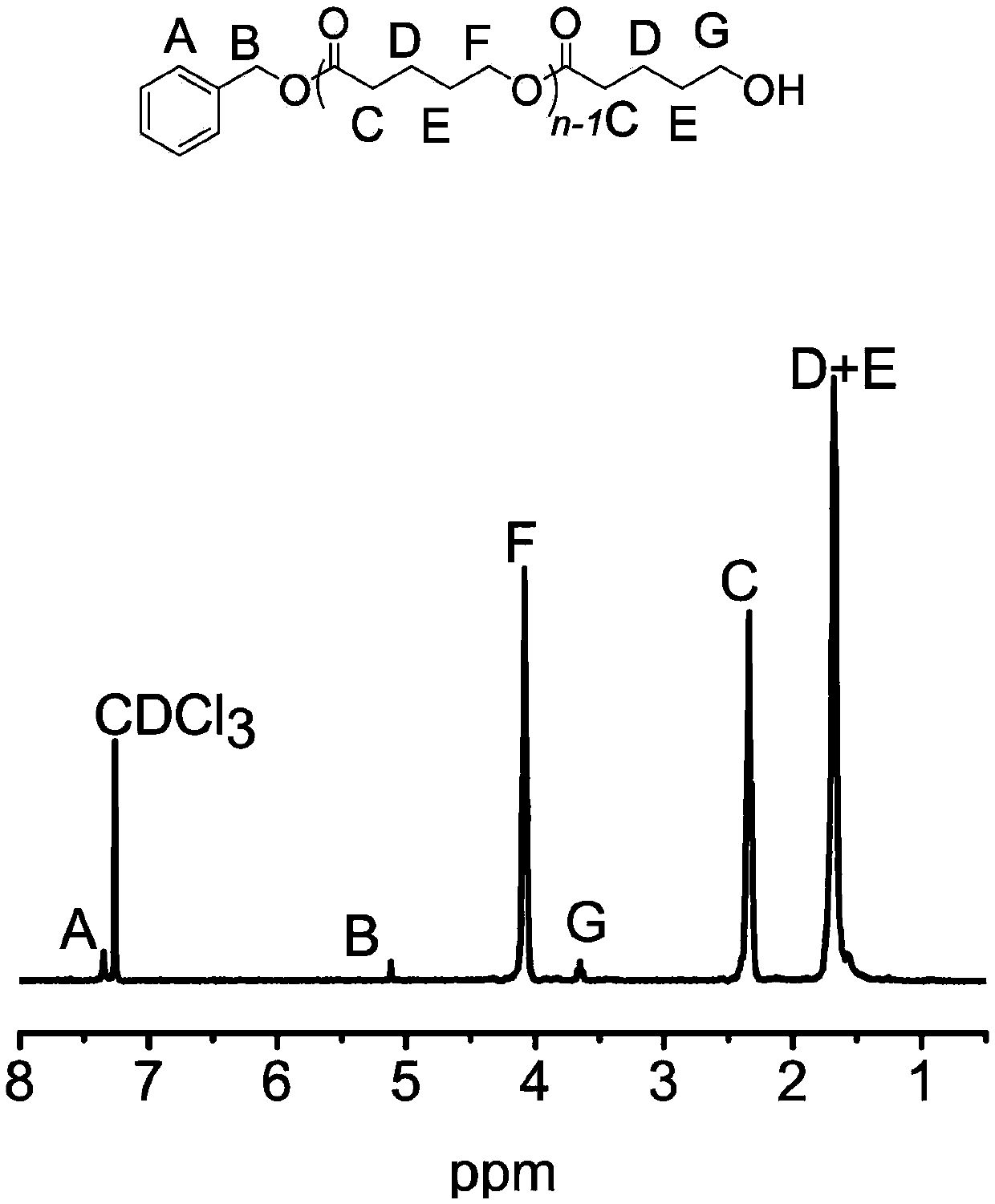
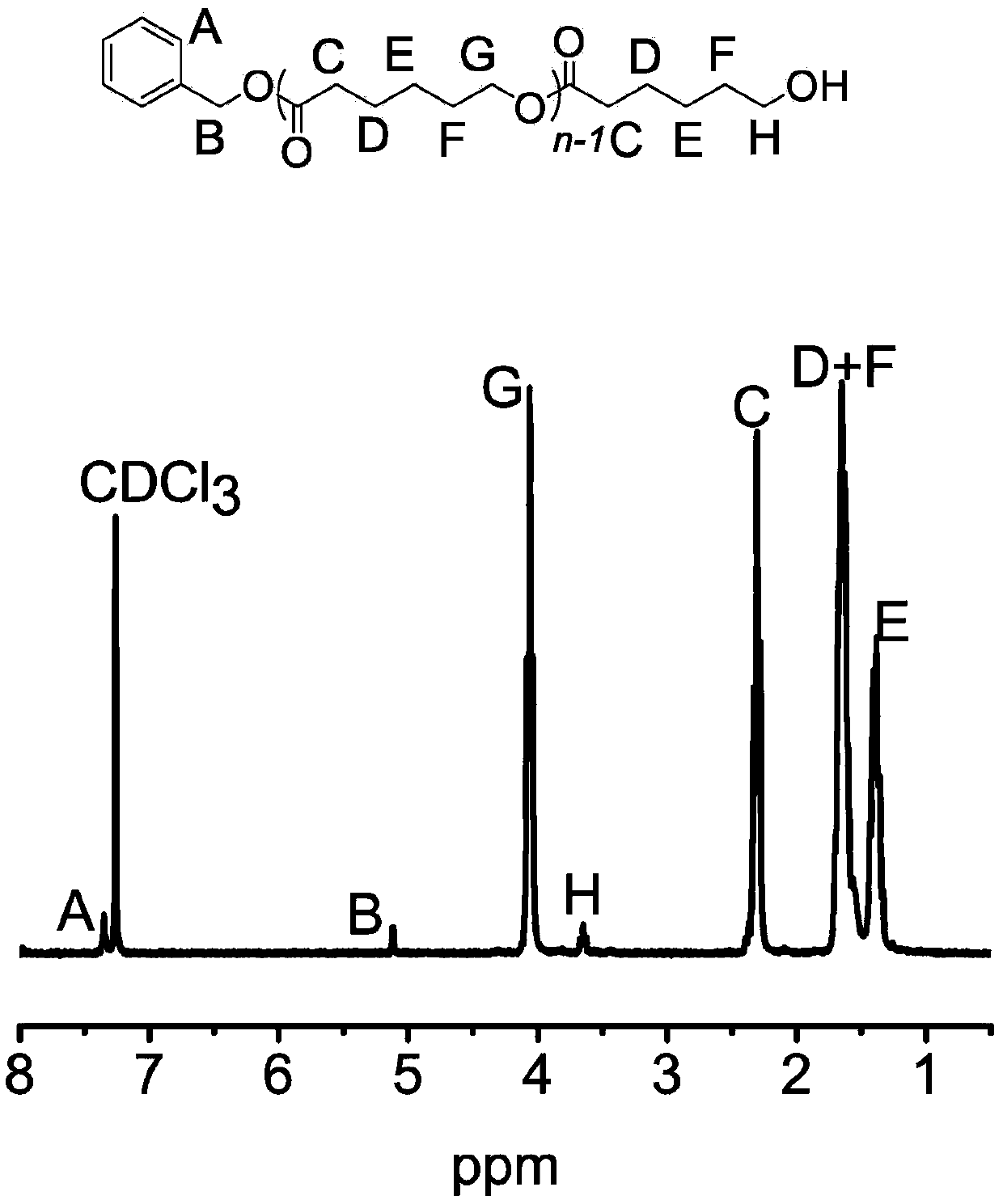
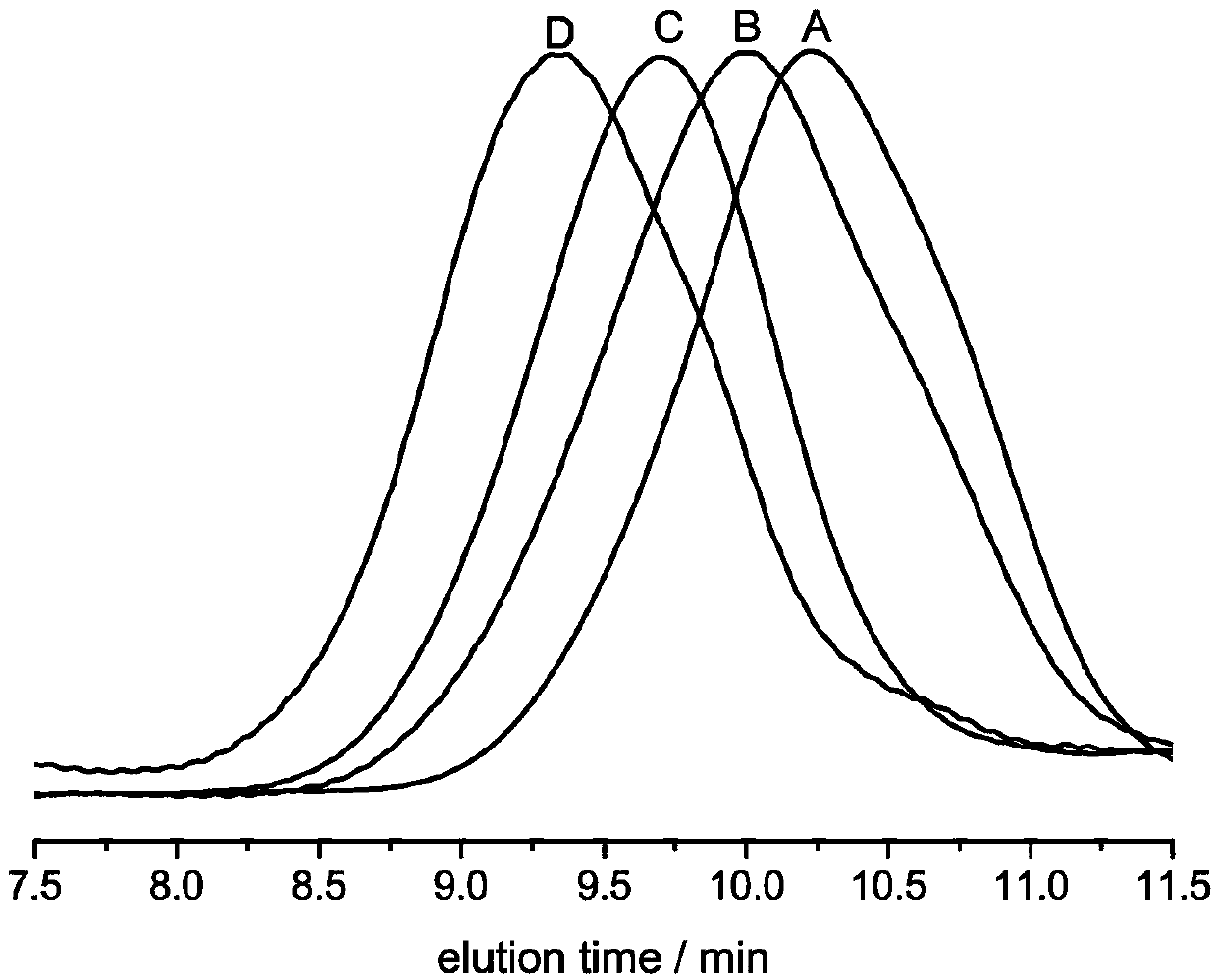
[0059] In a 5 mL polymerization tube, add valerolactone (0.1812 mL, 2 mmol), 2 mL of dichloromethane, thiourea TU 5 (0.033 g, 0.067 mmol), TFA trifluoroacetic acid (5 microliters, 0.067 mmol), 6.6 microliters (0.067 mmol) of benzyl alcohol were stirred at 20° C. for 37 hours. After the reaction was finished, the reaction was terminated with a small amount of triethylamine. The reaction solution was rotary evaporated, and the obtained crude product was dissolved in a minimum amount of dichloromethane, and then added into a cold methanol solution, and a polymer was precipitated. Centrifuge to obtain a white solid, which is transferred to a vacuum oven for drying. polymer structure through 1 HNMR identification, molecular weight and dispersion of the polymer were determined by GPC. After determination, the transformation rate of polymer is 99%, and productive rate is 61%, M n / M w is 1.30.
Embodiment 2
[0061] In a 5 mL polymerization tube, add caprolactone (0.2134 mL, 2 mmol), dichloromethane 2 mL, thiourea TU 5 (0.033 g, 0.067 mmol), TFA trifluoroacetic acid (5 microliters, 0.067 mmol), 6.6 microliters of benzyl alcohol (0.067 mmol) and stirred at 20° C. for 58 hours. After the reaction was finished, the reaction was terminated with a small amount of triethylamine. The reaction solution was rotary evaporated, and the obtained crude product was dissolved in a minimum amount of dichloromethane, and then added into a cold methanol solution, and a polymer was precipitated. Centrifuge to obtain a white solid, which is transferred to a vacuum oven for drying. polymer structure through 1 HNMR identification, molecular weight and dispersion of the polymer were determined by GPC. After determination, the transformation rate of polymer is 99%, and productive rate is 68%, M n / M w is 1.33.
Embodiment 3
[0063] In a 5 mL polymerization tube, add valerolactone (0.1812 mL, 2 mmol), 2 mL of dichloromethane, thiourea TU 5 (0.033 g, 0.067 mmol), TFA trifluoroacetic acid (5 microliters, 0.067 mmol), 6.6 microliters (0.067 mmol) of benzyl alcohol were stirred at 40° C. for 30 hours. After the reaction was finished, the reaction was terminated with a small amount of triethylamine. The reaction solution was rotary evaporated, and the obtained crude product was dissolved in a minimum amount of dichloromethane, and then added into a cold methanol solution, and a polymer was precipitated. Centrifuge to obtain a white solid, which is transferred to a vacuum oven for drying. polymer structure through 1 HNMR identification, molecular weight and dispersion of the polymer were determined by GPC. After determination, the transformation rate of polymer is 98%, and productive rate is 62%, M n / M w is 1.32.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com