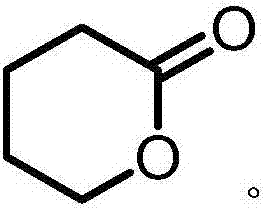
Method for manufacturing delta-valerolactone
A technology of valerolactone and alkyl hydroxyvalerate, which is applied in the field of manufacturing δ-valerolactone, and can solve the problems of low product yield, high price, and increased production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
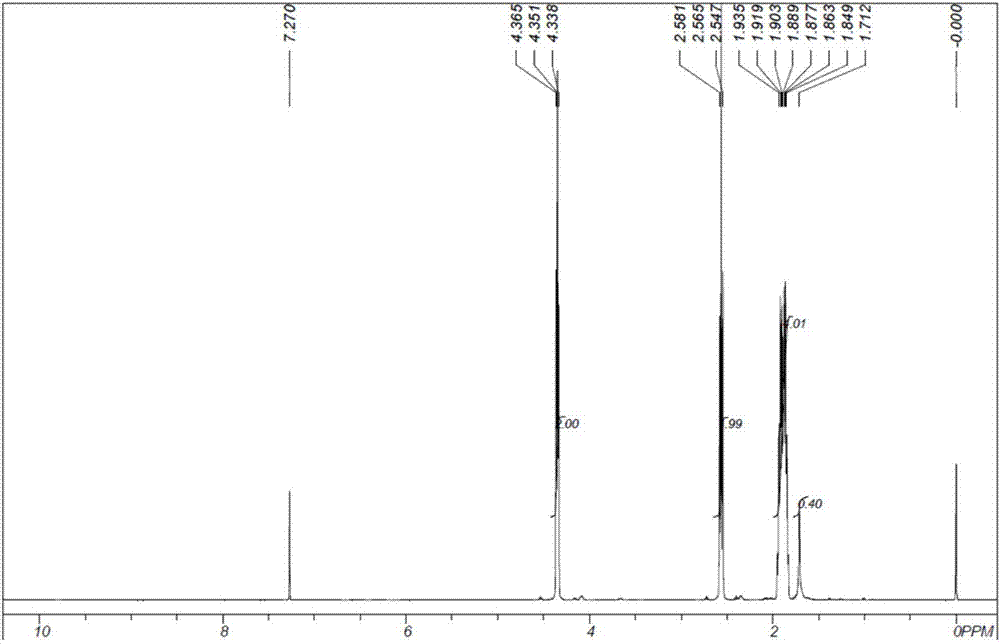
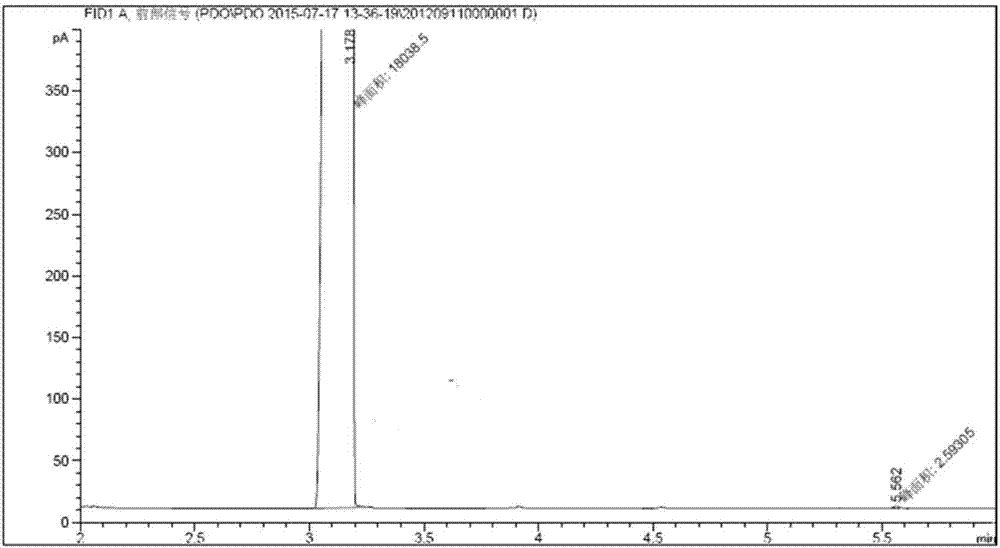
[0061] (1). The preparation of methyl 4,5-epoxyvalerate: 42.08g sodium hydroxide (1.01mol, 96%) and 185g methanol are placed in a 500ml four-necked flask, heated to methanol reflux temperature, and then dropwise Add 75.54g methyl acetate (1.01mol, 99%) and 92.50g epichlorohydrin (1.00mol, 99%) dropwise to the liquid funnel at the same time, the time for adding drops is 5h, keep the reaction temperature as the methanol reflux temperature, after the feed is finished, Continue the heat preservation reaction for 1h to obtain 394.99g of the reaction suspension containing white powder precipitate, and use a 50μm filter to filter under reduced pressure at 0.03MPa to obtain 330.89g of the filtrate. The gas chromatography analysis product content is 38.11wt.%. The yield of oxychloropropane is 97.00%.
[0062] (2). Preparation of methyl 5-hydroxyvalerate: The synthetic reaction of methyl 5-hydroxyvalerate was carried out in a stainless steel tubular trickle bed reactor with an inner dia...
Embodiment 2
[0073] (1). The preparation of ethyl 4,5-epoxyvalerate: 218.75g sodium hydroxide (5.25mol, 96%) and 1850.00g ethanol are placed in a 5000ml four-necked flask, heated to ethanol reflux temperature, and then Add 466.95g of ethyl acetate (5.25mol, 99%) and 462.50g of epichlorohydrin (5.0mol, 99%) dropwise to the dropping funnel at the same time, the time for adding is 3h, keep the reaction temperature at the reflux temperature of ethanol, and the feeding is finished Afterwards, the insulation reaction was continued for 1h, and 2987.34g of the reaction suspension containing white powder precipitate was obtained, which was filtered under reduced pressure at 0.03MPa using a 50 μm filter to obtain 2675.60g of the filtrate, and the gas chromatography analysis product content was 26.29wt.%. The yield of oxychloropropane is 97.71%.
[0074] (2). Preparation of ethyl 5-hydroxyvalerate: reactor and catalyst are the same as in Example 1.
[0075] The filtrate containing ethyl 4,5-epoxypen...
Embodiment 3
[0085] (1). The preparation of 4,5-epoxypropyl valerate: 214.58g sodium hydroxide (5.15mol, 96%) and 1387.50g propanol are placed in a 5000ml four-necked flask, heated to propanol reflux temperature, Then adopt dropping funnel to dropwise add 530.97g propyl acetate (5.15mol, 99%) and 462.50g epichlorohydrin (5.0mol, 99%) simultaneously, dropwise time is 4h, keeps reaction temperature to be propanol reflux temperature, After the feed was completed, the heat preservation reaction was continued for 1 hour, and 2587.62 g of the reaction liquid containing a white powder precipitate was obtained, which was filtered with a 50 μm filter under reduced pressure at 0.03 MPa to obtain 2283.15 g of the filtrate, and the product content of gas chromatography analysis was 33.73 wt.%. , The yield is 97.48% in terms of epichlorohydrin.
[0086] (2). Preparation of 5-hydroxypropyl valerate: reactor and catalyst are the same as in Example 1.
[0087] The filtrate containing 4,5-epoxypropyl vale...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com