Preparation method of delta-valerolactone
A technology of valerolactone and alkyl acetate, which is applied in the field of preparation of δ-valerolactone, can solve problems such as disastrous consequences, difficulty in obtaining, sensitive reaction conditions, etc., achieve good physical properties, avoid safety hazards, and react The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Catalyst preparation:
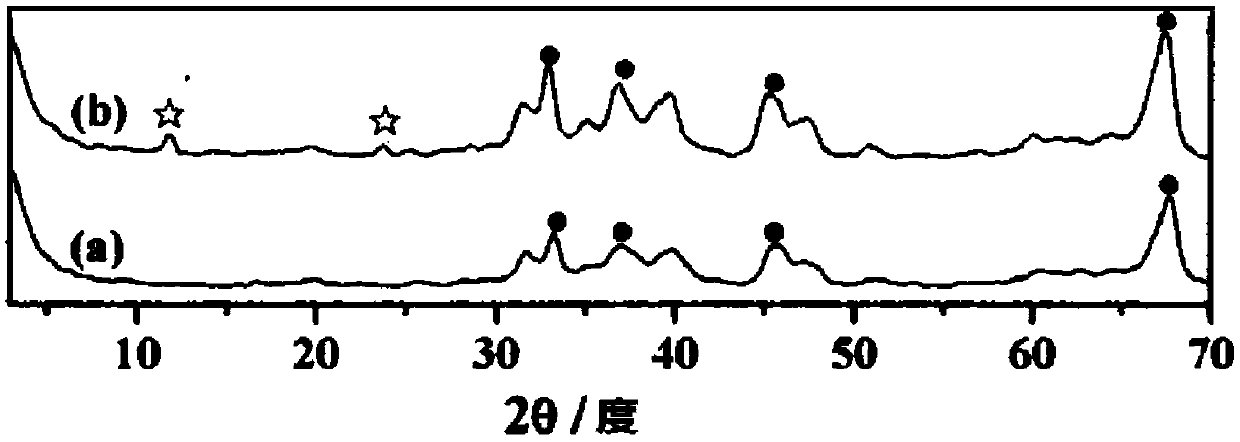
[0051] Weigh 102g of Al 2 o 3 (1mol), mix it with 100ml of aqueous urea (CO(NH 2 ) 2 : 0.005mol) mixed evenly, placed in a 2L autogenous autoclave, kept at 90°C for 24h, then added 700ml of Mg(NO 3 ) 2 (0.02mol) aqueous solution, and heated up to 130°C, kept for 24h, washed with deionized water, dried at 120°C, and then roasted at 450°C for 8h to obtain LDHs-modified alumina composite MaterialLDHs-Al 2 o 3 .
[0052] Prepare a certain concentration of sodium phosphate (wherein the amount of sodium phosphate substance is 0.1mol) aqueous solution, then 100g of the LDHs-Al prepared above 2 o 3 Put it into the sodium phosphate aqueous solution, shake it well, put it into the constant temperature oscillator in the water bath for 24 hours, keep the temperature of the water bath at about 70°C during this period, and the shaking rate is 130-140r / min. Then keep this temperature and continue to shake, add dropwise lithium chloride aqueous solutio...
Embodiment 2
[0062] Catalyst preparation:
[0063] With embodiment 1, difference is that alkaline substance CO(NH 2 ) 2 The dosage is 0.014mol, Mg(NO 3 ) 2 The dosage is 0.08mol, the dosage of sodium phosphate is 0.58mol, the dosage of lithium chloride is 1.77mol, the prepared catalyst is 166.53g, and the loading capacity of the active component lithium phosphate is 39.95%.
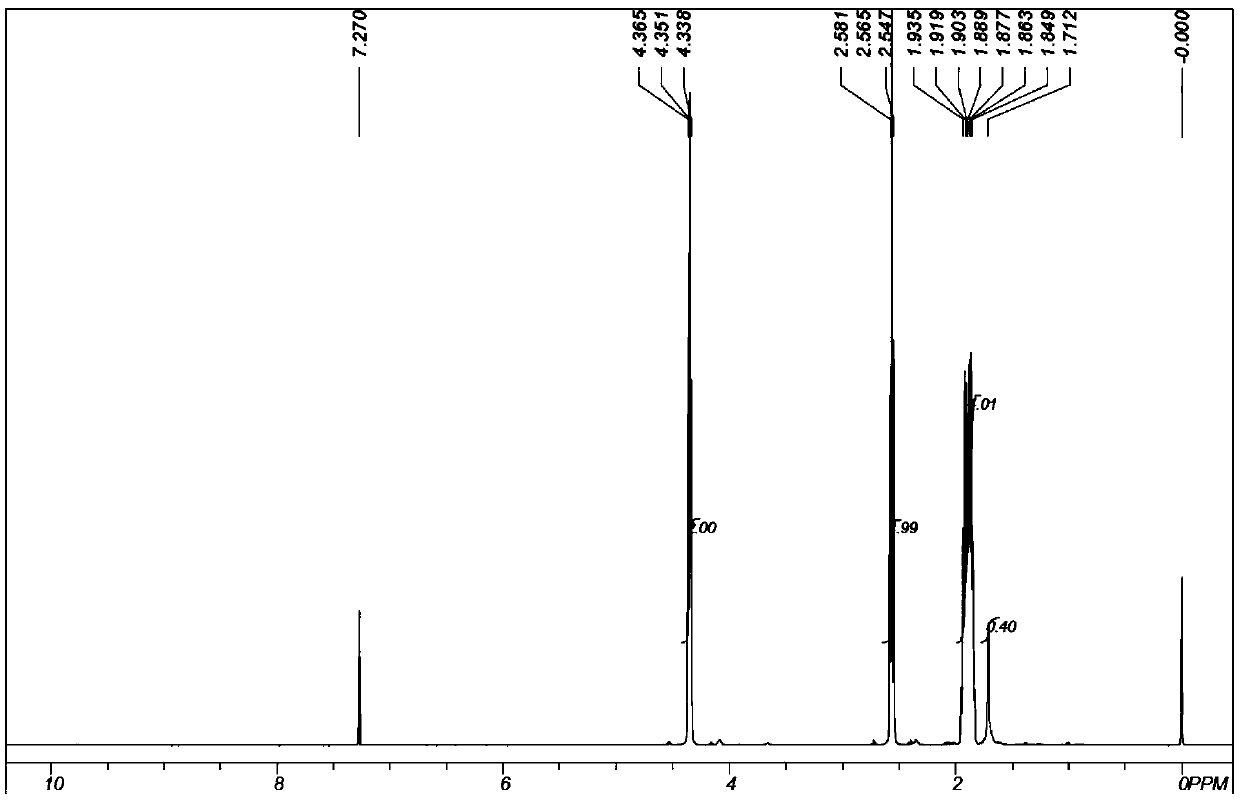
[0064] Preparation of δ-valerolactone:
[0065] Reactor and catalyst loading mode, loading amount are the same as embodiment 1.
[0066] Keep the reaction section of the catalytic rectification tower and the jacket temperature of the stripping section at 250°C, and continuously feed ethyl acetate and propylene oxide through the feed pump, wherein the liquid space velocity of propylene oxide WHSV=5.0g / gcat / h, acetic acid The molar ratio of ethyl ester to propylene oxide is 1.05:1, and the reflux ratio of the catalytic rectification tower is 10. The feed in the tower is used, and low-boiling compounds such as ethan...
Embodiment 3
[0072] Catalyst preparation:
[0073] With embodiment 1, difference is that alkaline substance CO(NH 2 ) 2 The dosage is 0.01mol, Mg(NO 3 ) 2 The dosage is 0.05mol, the dosage of sodium phosphate is 0.39mol, the dosage of lithium chloride is 1.18mol, the prepared catalyst is 145.07g, and the loading capacity of the active component lithium phosphate is 31.07%.
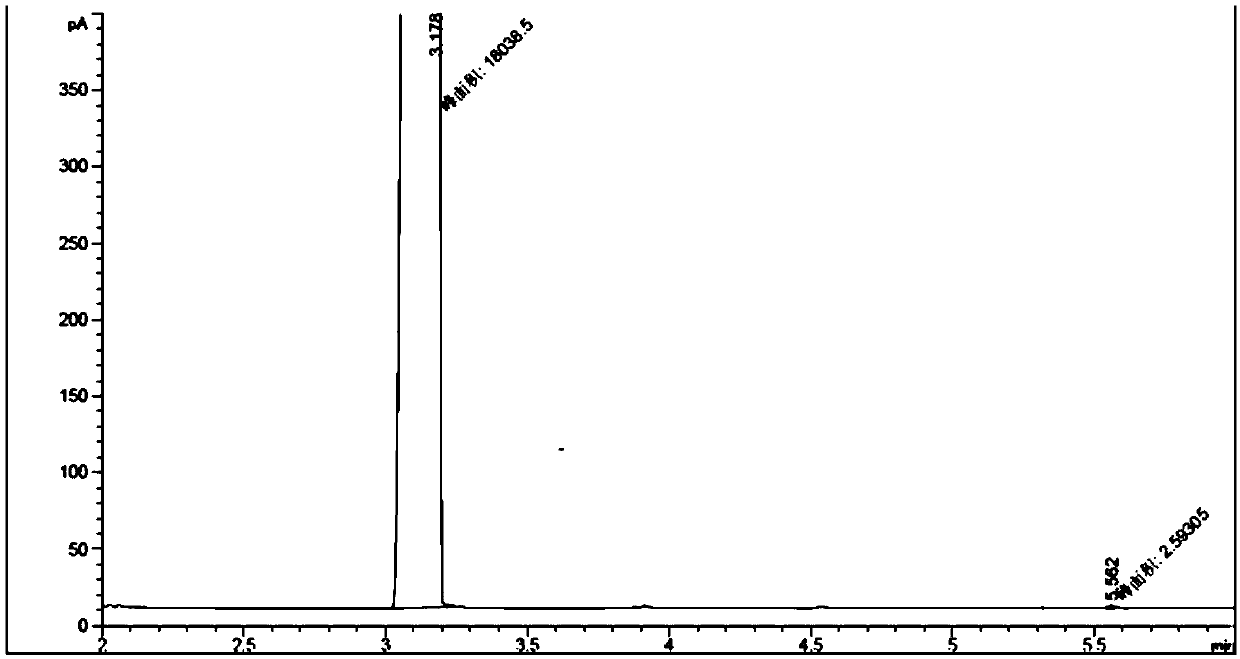
[0074] Preparation of δ-valerolactone:
[0075] Reactor and catalyst loading mode, loading amount are the same as embodiment 1.
[0076] Keep the reaction section of the catalytic rectification tower and the jacket temperature of the stripping section at 215°C, continuously feed propyl acetate and propylene oxide through the feed pump, wherein the liquid space velocity of propylene oxide WHSV=3.0g / gcat / h, acetic acid The molar ratio of methyl ester to propylene oxide is 1.03:1, the reflux ratio of the catalytic rectification tower is 5, the feed in the tower is adopted, the low boiling point compounds such as prop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com