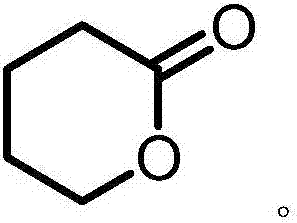
Method for producing DVL (delta-valerolactone)
A technology of valerolactone and aldehyde valeronitrile, which is applied in the field of manufacturing δ-valerolactone, can solve problems such as limitations, disasters, and difficult product separation, and achieve the effects of mild reaction conditions, less environmental pollution, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
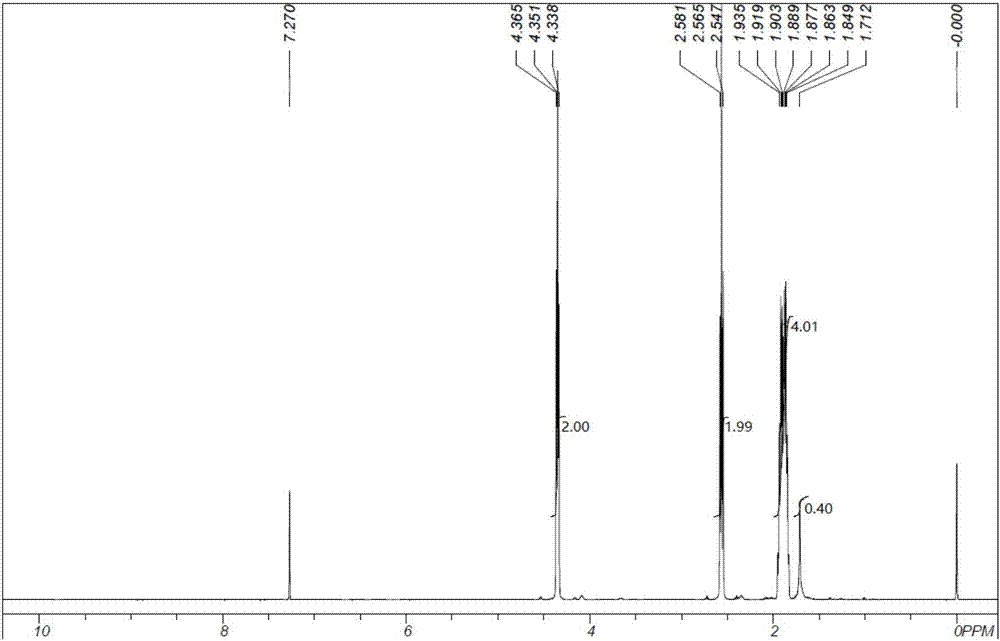
[0041] (1). Preparation of 5-formyl valeronitrile: 41.88g (1.01mol, 99%) of acetonitrile is placed in a 250ml four-necked flask, warmed up to reflux temperature, and then 0.1822g (0.0005mol, 0.0005mol, 25%) tetramethylammonium hydroxide solution and 56.60g of acrolein (1.00mol, 99%), the dropwise addition time is 5h, the reaction temperature is kept at reflux temperature, and the insulation reaction is continued for 1h after the feeding is completed, and 98.06g of the reaction solution is obtained .
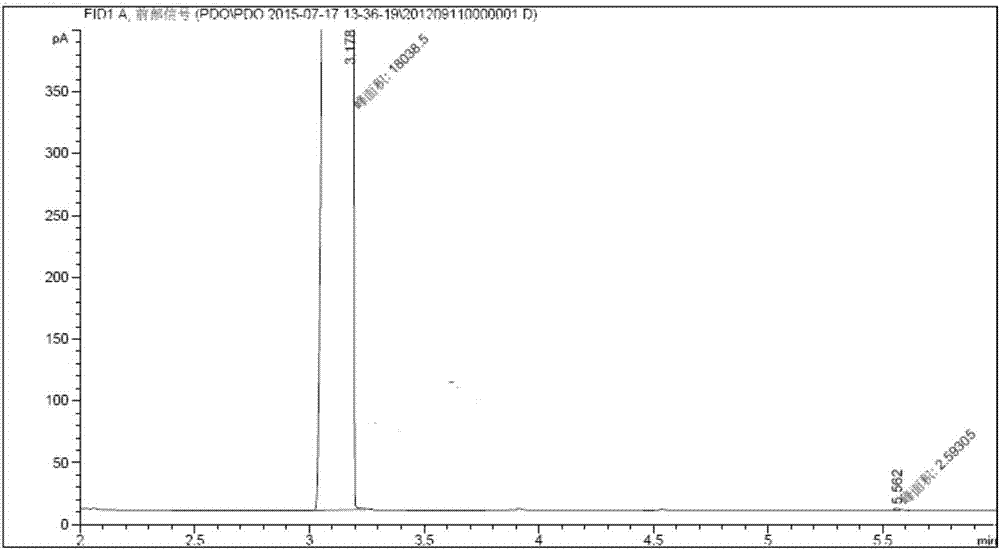
[0042] The reaction solution was subjected to vacuum distillation at 5.0KPa and 130°C after removal of light components at normal pressure and 100°C, and 94.93g of the product at the temperature of 101-104°C / 5.0KPa was collected, and the purity of the gas chromatographic analysis was 99.0%. The yield of the product based on acrolein is 96.89%.
[0043] (2). Preparation of 5-hydroxyvaleronitrile: The synthetic reaction of 5-hydroxyvaleronitrile is carried out in a trickle bed rea...
Embodiment 2
[0054] (1). Preparation of 5-formyl valeronitrile: 434.8g (10.5mol, 99%) of acetonitrile is placed in a 2500ml four-necked flask, warmed up to reflux temperature, and then 36.44g (0.1mol, 25%) tetramethylammonium hydroxide solution and 566.0g acrolein (10.0mol, 99%), the dropwise addition time is 2h, the reaction temperature is kept at the reflux temperature, and the insulation reaction is continued for 1h after the feeding is completed, and 1029.46g of the reaction solution is obtained .
[0055] The product purification was the same as in Example 1, and 955.33 g of a product with a purity of 99.2% was obtained, and the yield of the final product calculated as acrolein was 97.70%.
[0056] (2). Preparation of 5-hydroxyvaleronitrile: The reactor, catalyst and activation process are the same as in Example 1.
[0057] The 5-formyl valeronitrile prepared by reaction step 1) enters continuously through a feed pump, liquid space velocity WHSV=5.0g / gcat / h, hydrogen and 5-formyl val...
Embodiment 3
[0062] (1). Preparation of 5-formyl valeronitrile: 424.5g (10.25mol, 99%) of acetonitrile is placed in a 2500ml four-neck flask, warmed up to reflux temperature, and then 9.11g (0.025mol, 9.11g, 0.025mol, 25%) tetramethylammonium hydroxide solution and 566.0g acrolein (10.0mol, 99%), the dropwise addition time is 4h, keep the reaction temperature at the reflux temperature, continue the insulation reaction for 1h after feeding, and obtain 992.91g reaction liquid .
[0063] The product purification was the same as in Example 1, and 954.34 g of a product with a purity of 99.1% was obtained, and the yield of the final product was 97.50% based on acrolein.
[0064] (2). Preparation of 5-hydroxyvaleronitrile: The reactor and catalyst activation process are the same as in Example 1, and the catalyst is copper-silicon dioxide (Cu: 21.55wt%, based on the total weight of the catalyst based on copper element) catalyst.
[0065] The 5-formyl valeronitrile prepared by reaction step 1) ent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com