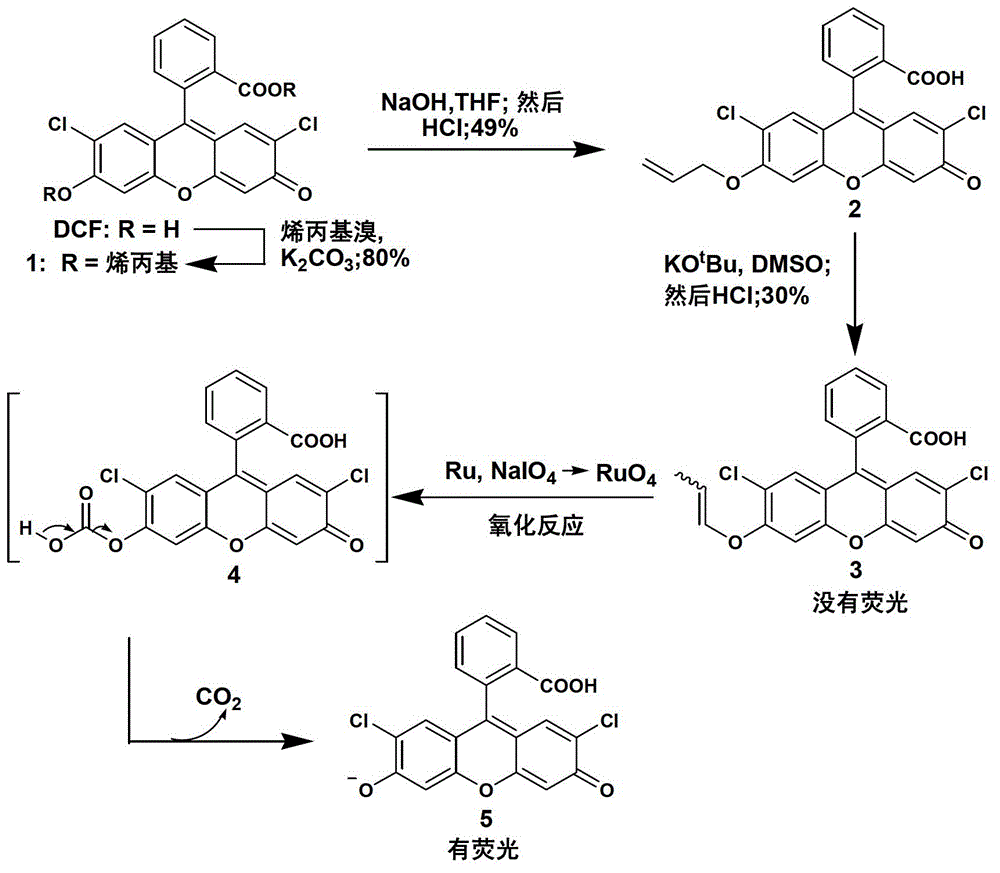
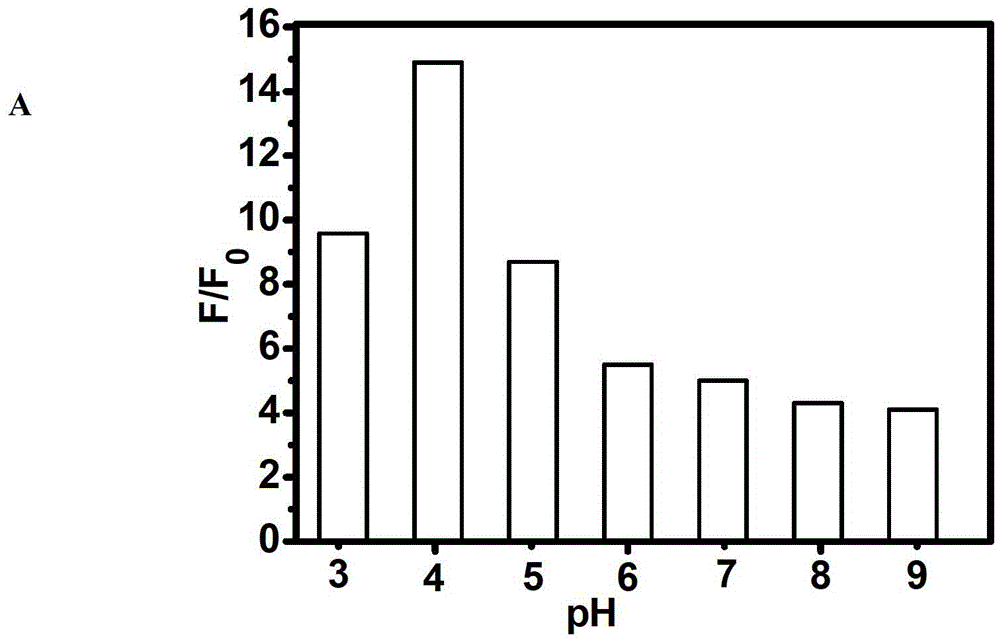
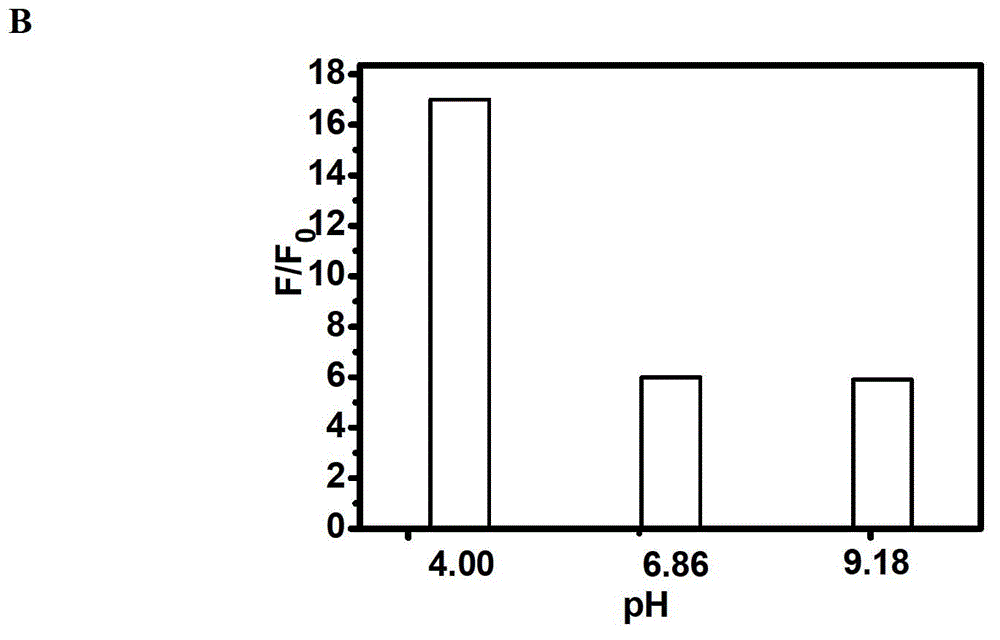
Fluorescent compound and application in ruthenium detection
A fluorescent compound and compound technology, applied in the field of fluorescent compounds, can solve the problems of complex sample pretreatment process, high cost, and difficulty in popularization and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The synthetic method of embodiment 1 compound 1
[0057] Under the condition of nitrogen protection, add 3g DMF (2,7-dichlorofluorescein), 2.6g anhydrous potassium carbonate and 40mL dry DMF to a dry 100mL single-necked bottle, add a magnet, heat and stir in an oil bath at 50°C , then add 2.26mL allyl bromide under the protection of nitrogen, continue stirring for 12 hours, stop the reaction, add 250mL deionized water after cooling, and then extract three times with 300ml ethyl acetate, the collected organic phase is washed with water and saturated with chlorine Wash with sodium chloride solution, dry the organic phase over anhydrous sodium sulfate, filter, rotary evaporate, and recrystallize the product with n-hexane to finally obtain 2.8 g of orange-red compound 1 as a solid, with a yield of 80%. Chemical equations are shown in figure 1 middle.
Embodiment 2
[0058] The synthetic method of embodiment 2 compound 2
[0059]Add 1g of compound 1, 0.66g of sodium hydroxide and 40mL of tetrahydrofuran into a dry 100mL single-necked bottle, add a magneton and heat and stir in an oil bath at 70°C for 6 hours, adjust the pH to 1 with 3M dilute hydrochloric acid after cooling, and then use 30 mL of ethyl acetate was extracted, the organic phase was washed with water and saturated brine, dried over anhydrous sodium sulfate, filtered, and rotary evaporated. The crude product was purified by column chromatography to obtain 450 mg of a yellow-white product. Chemical equations are shown in figure 1 middle. 1 H NMR (400MHz, CDCl 3 ,293K)δ8.12–8.01(dd,1H),7.79–7.60(m,2H),7.20–7.12(m,1H),6.97–6.89(m,1H),6.85–6.78(m,1H), 6.76–6.68(m,2H),6.16–5.98(m,1H),5.50(dd,J=17.3,1.4Hz,1H),5.37(dd,J=10.6,1.3Hz,1H),4.67(d, J=5.0Hz,2H).
Embodiment 3
[0060] The synthetic method of embodiment 3 compound 3
[0061] Under the condition of nitrogen protection, 150 mg of potassium tert-butoxide and 300 mg of compound 2 were added to a 100 mL single-necked bottle, and finally 10 mL of DMSO was added, and a magnet was added and heated and stirred in an oil bath at 80°C for 12 hours. After cooling, adjust the pH to 1 with 3M dilute hydrochloric acid, then add 50 mL of deionized water, extract with 30 mL of ethyl acetate, wash the organic phase with water and saturated brine, dry over anhydrous sodium sulfate, filter, and rotary evaporate, and the crude product is passed through the column Purified by chromatography to finally obtain 45 mg of yellow product. Chemical equations are shown in figure 1 middle. 1 H NMR (400MHz, CDCl 3 ,293K)δ8.07(d,J=7.2Hz,1H),7.71(ddd,J=19.0,11.1,6.9Hz,2H),7.17(d,J=7.4Hz,1H),6.92(d,J =6.5Hz,2H),6.77(s,1H),6.72(s,1H),6.47–6.31(m,1H),5.20–5.07(m,1H),1.74(dd,J=6.9,1.6Hz, 3H). 13 C NMR (101MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com