Delta-valerolactone-based polymer and preparation method and application thereof
A technology of valerolactone and polymer, applied in the field of delta-valerolactone-based polymer and its preparation, can solve problems such as high toxicity and low transfection efficiency, and achieve easy control, simple preparation method and good compatibility sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0051] A delta-valerolactone-based polymer characterized in that the delta-valerolactone-based polymer has the structure in [Chemical Formula 1] shown below:
[0052] [chemical formula 1]
[0053]
[0054] Among them, R 2 for and One of them, n is greater than or equal to 1, and m is greater than or equal to 0. n and m are varied according to the ratio of the two parts.
[0055] [Chemical formula 1] The specific synthetic route of the polymer is:
[0056]
[0057] Wherein, the compound of [chemical formula 2] is compound 1 in the synthetic route, and in the compound of [chemical formula 3], a is 2, 6, 10 or 14, and the corresponding compounds in the synthetic route are 2, 3, 4, 5;
[0058] The obtained δ-valerolactone-based polymer has a number-average molecular mass in the range of 2,000-10,000, and the structural terminal is a hydroxyl group.
[0059] Concrete synthetic steps are as follows:
[0060] Step 1. Weigh the exocyclic α, β-unsaturated δ-valerolactone...
example 2
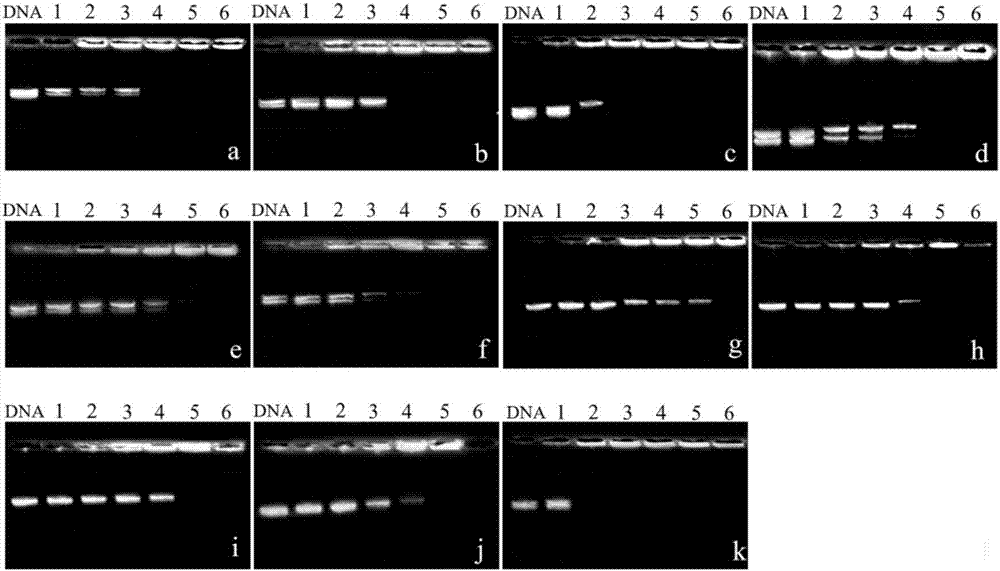
[0075] Prepare solutions of different concentrations of polymers (B1-B6, C1-C4, P1) respectively, place the solutions of different concentrations of polymers and PGL-3 plasmid DNA (9μg / mL) in a water bath at 37°C for 1 hour, and carry out DNA agarose gel retardation experiment obtained the gel retardation results of different concentrations of polymers (B1-B6, C1-C4, P1) on PGL-3DNA.
[0076] figure 1 a~1k are the agarose gel retardation experiment results of the PGL-3DNA of the polymers B1-B6, C1-C4, and P1 of the present invention respectively; wherein, figure 1 a is the agarose gel retardation experiment diagram of polymer B1 to pGL-3 in Example 1 of the present invention; figure 1 b is the agarose gel retardation experiment diagram of polymer B2 to pGL-3 in Example 1 of the present invention; figure 1 c is the agarose gel retardation experiment diagram of polymer B3 to pGL-3 in Example 1 of the present invention; figure 1 d is the agarose gel retardation experiment diag...
example 3
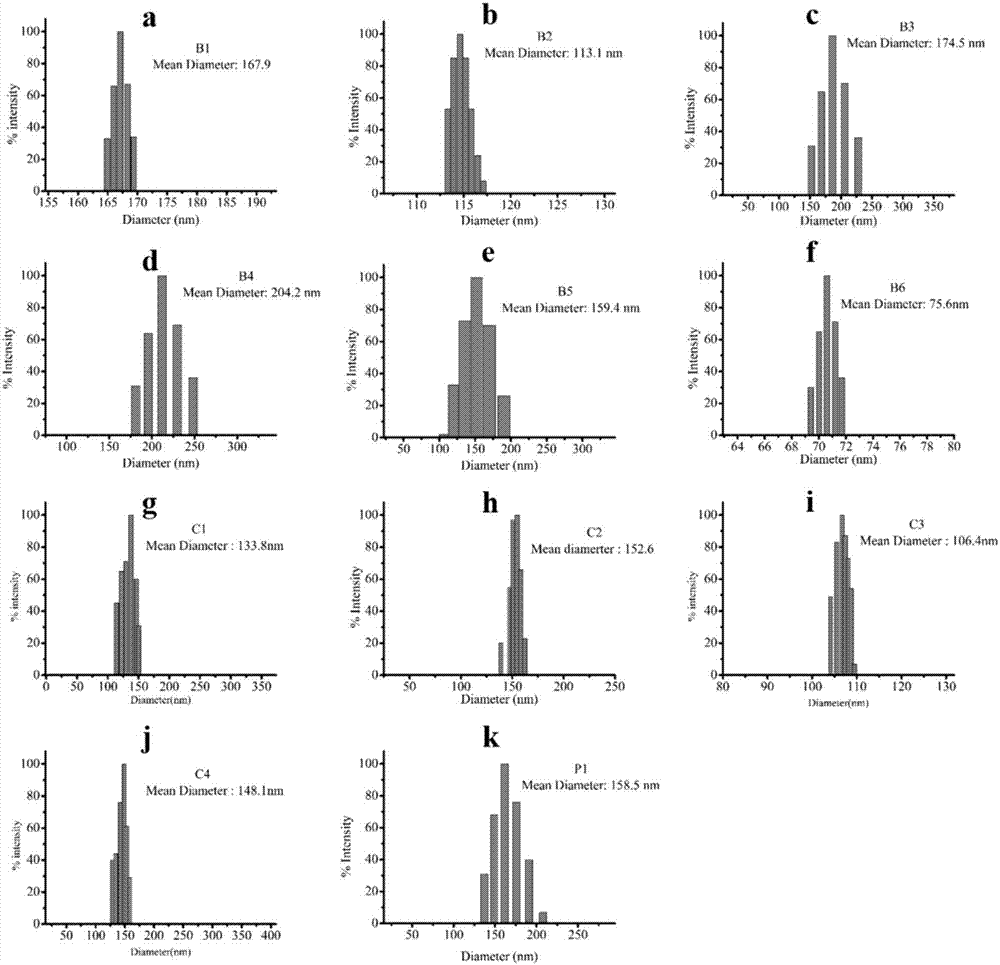
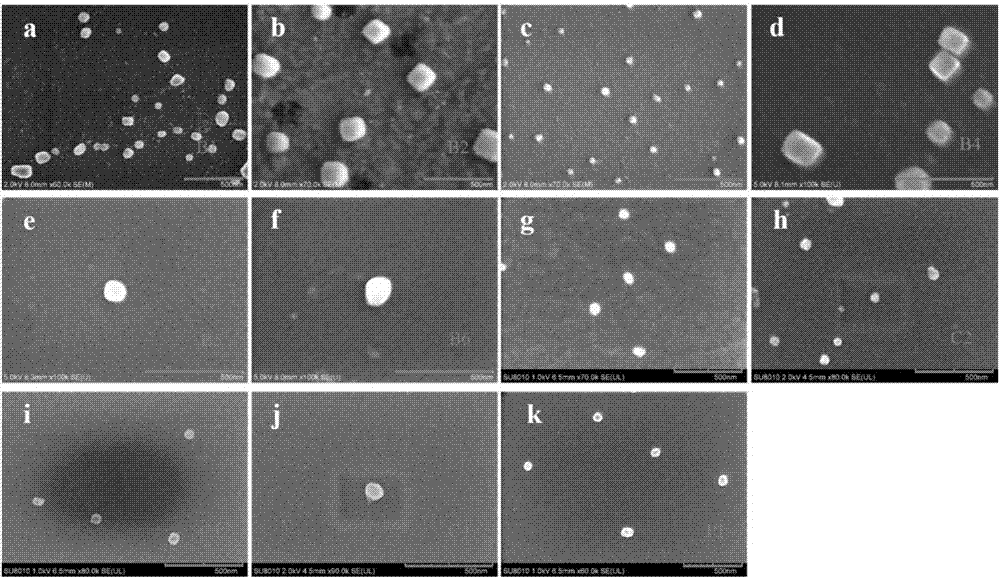
[0079] Prepare solutions of different concentrations of polymers (B1-B6, C1-C4, P1), put the solutions of different concentrations of polymers and PGL-3 plasmid DNA (9μg / mL) in a water bath at 37°C for 1 hour, and carry out In the study of dynamic laser light scattering technology, we studied the particle size of the aggregated polymer and DNA.
[0080] figure 2 a~2k are the dynamic light scattering experiment results of PGL-3DNA of polymers B1-B6, C1-C4, and P1 of the present invention, respectively; figure 2 a is a dynamic light scattering particle size diagram of a complex of polymer B1 and plasmid DNA according to the present invention; figure 2 B is the dynamic light scattering particle size diagram of the complex of polymer B2 and plasmid DNA according to the present invention; figure 2 c is the dynamic light scattering particle size diagram of the complex of polymer B3 and plasmid DNA according to the present invention; figure 2 D is the dynamic light scattering...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com