Macrocyclic polyamine compound based on dedpp-2tpa and its preparation method and application
A technology of DEDPP-2TPA and macrocyclic polyamine, applied in the field of DEDPP-2TPA-based macrocyclic polyamine compound and its preparation, can solve the problem of low transfection performance and achieve the effect of facilitating the transfection mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A kind of macrocyclic polyamine compound based on DEDPP-2TPA, the structural formula (I) of said compound is as follows:
[0053]
[0054] In the formula (I), R is a macrocyclic polyamine [12]aneN 3 Structural unit, n is a positive integer.
[0055] Among them, when R is When n=4, it is recorded as compound 1;
[0056] when R is When n=6, it is recorded as compound 2;
[0057] when R is When n=8, it is recorded as compound 3;
[0058] Concrete synthetic route is as follows:
[0059]
[0060] Concrete synthetic steps are as follows:
[0061] 1), Compound 4 (1.1g, 3mmol), TPA-B(OH) 2 (2.17g, 7.5mmol), Pd(PPh 3 ) 4 (0.312g, 0.27mmol) into the two-necked bottle, nitrogen protection, inject anaerobic THF 100mL and KCO 3 Aqueous solution (2M, 50mL), reflux at 80°C for 8h, remove THF under reduced pressure, extract and dry with DCM, use petroleum ether / ethyl acetate (v / v=15:1) as eluent, and separate by column chromatography to obtain a yellow solid Compou...
Embodiment 2
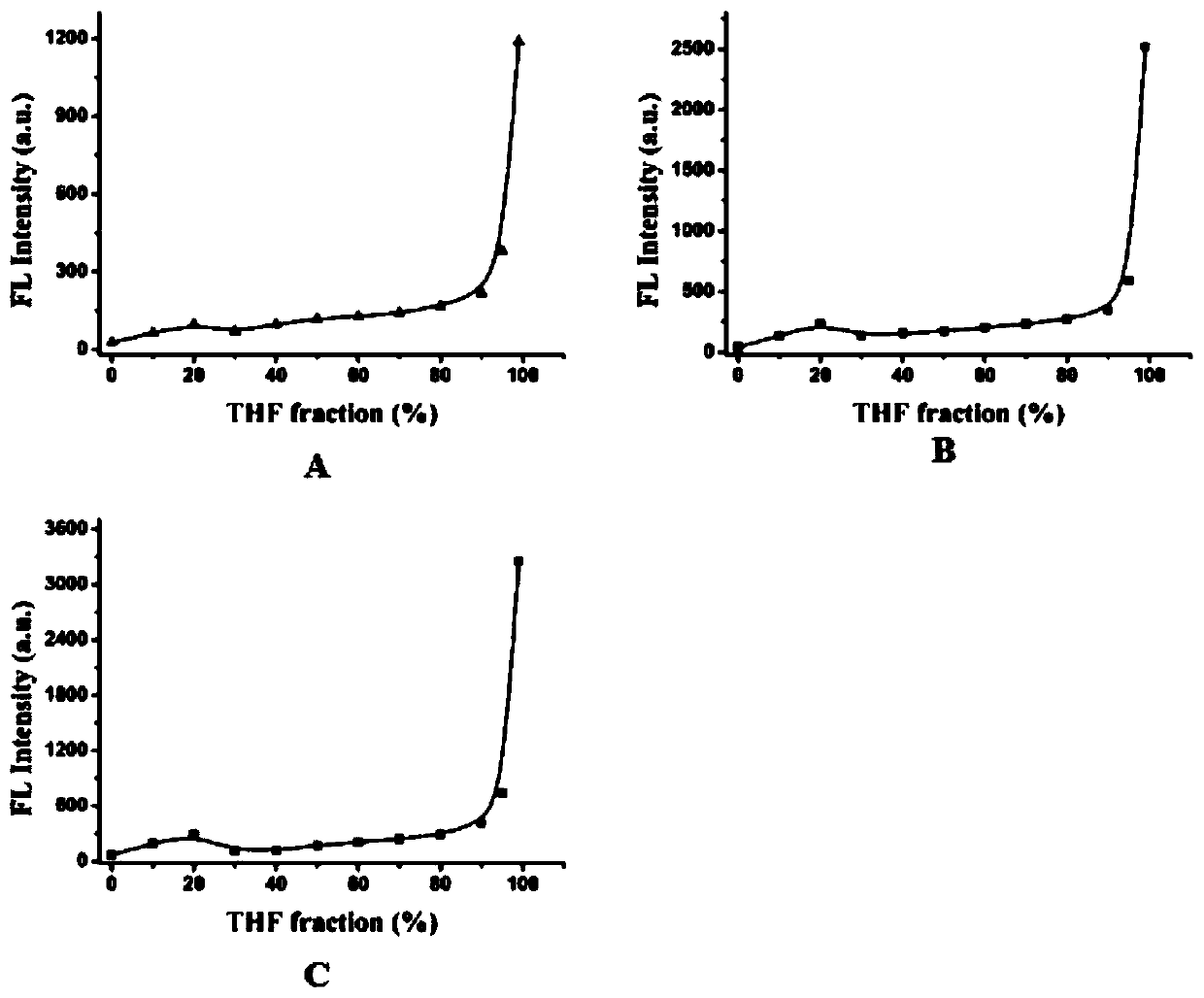
[0095] Prepare solutions with different proportions (0-99%) of tetrahydrofuran and water, add compounds 1, 2 and 3 to prepare a solution (10 μM) with the same concentration, and measure the fluorescence emission intensity of compounds 1, 2 and 3 in different solvent ratios; The maximum value of the intensity and the change trend of the proportion of THF are plotted to obtain figure 1 A~1C, in figure 1 In A to 1C, the X-axis represents the concentration of tetrahydrofuran, and the Y-axis represents the fluorescence intensity. Depend on figure 1 It can be concluded that the macrocyclic polyamine compound based on DEDPP-2TPA of the present invention has excellent AIE performance.
Embodiment 3
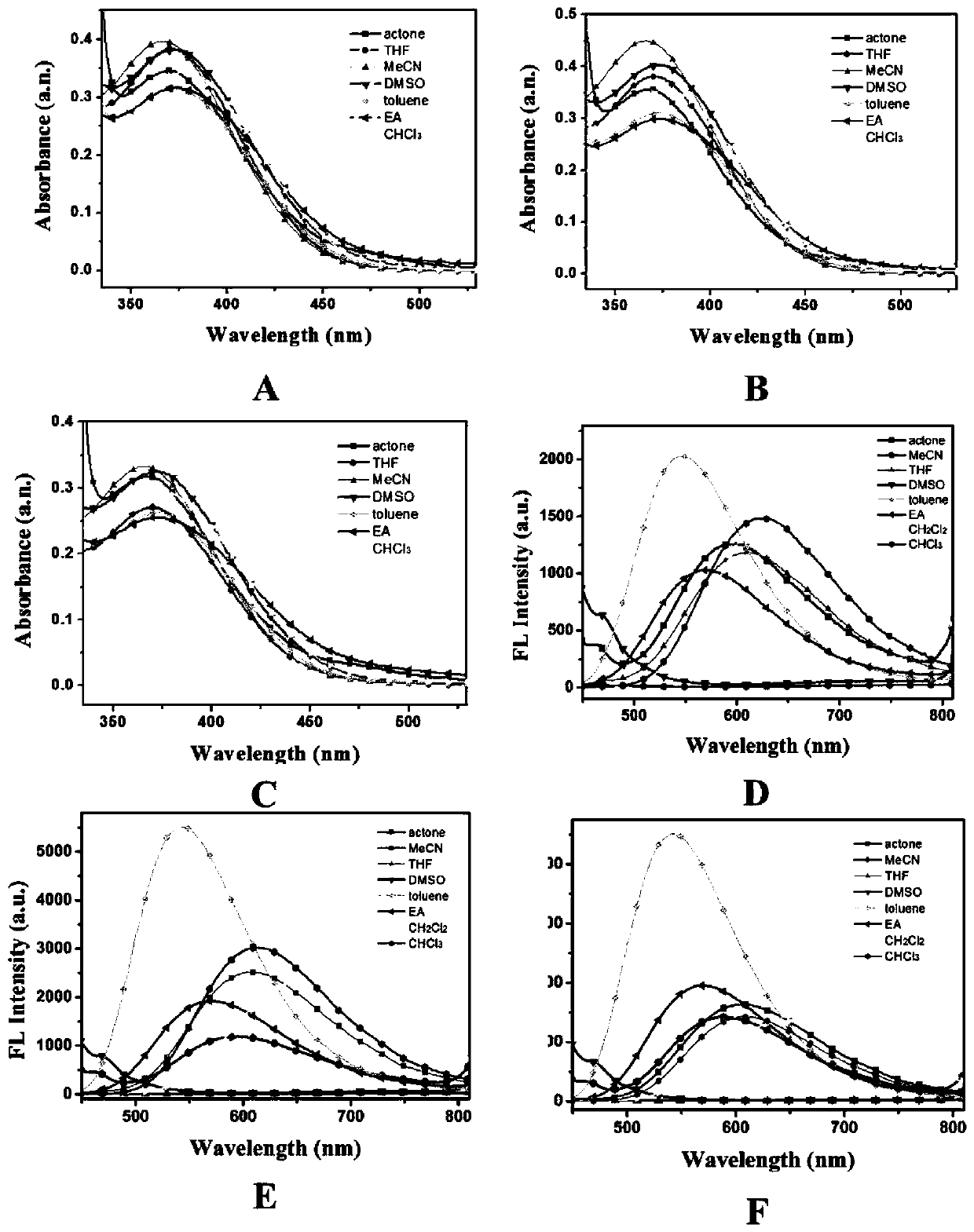
[0097] Compounds 1, 2 and 3 were prepared at 10 μM in different solvents, and their UV absorption and fluorescence emission were tested, and the spectrum was processed to obtain figure 2 A~2F. Depend on figure 2 It can be concluded that the DEDPP-2TPA-based macrocyclic polyamine compound of the present invention has the characteristics of large Stoke shift, long-wave excitation and low self-luminescence.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com