A kind of hyperbranched polysiloxane fluorescent material and preparation method
A polysiloxane and fluorescent material technology, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of weak fluorescence intensity and low quantum yield, and achieve high quantum yield, good biodegradability, and abundant synthesis. effect of the method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
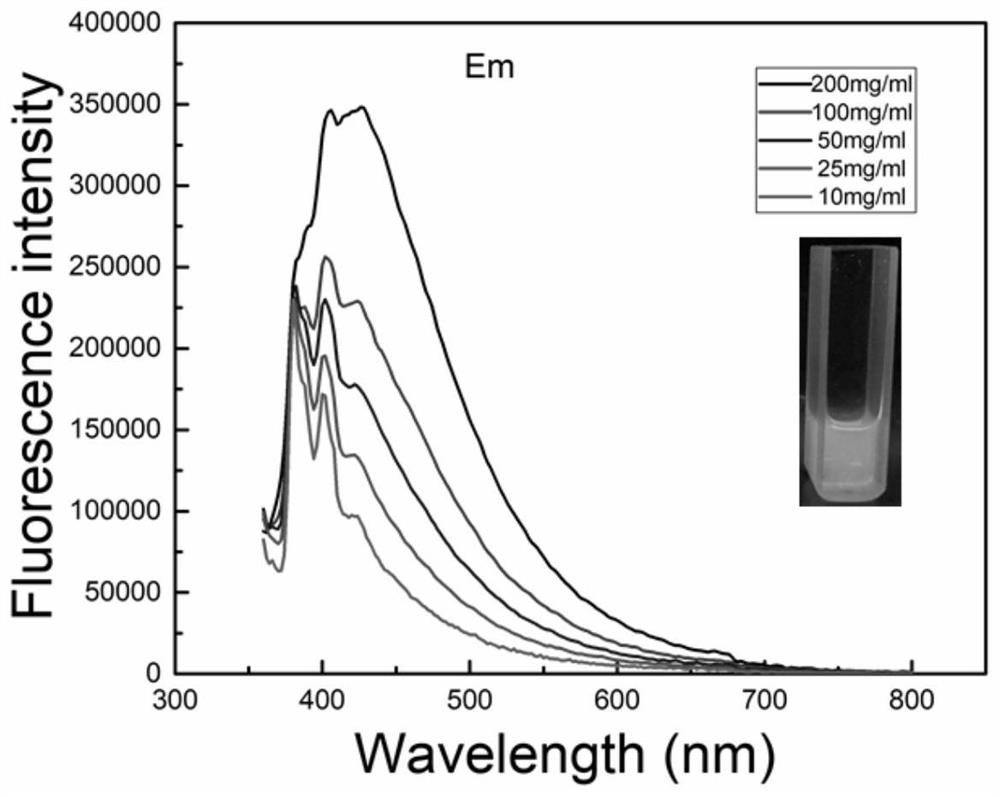
Examples
example 1
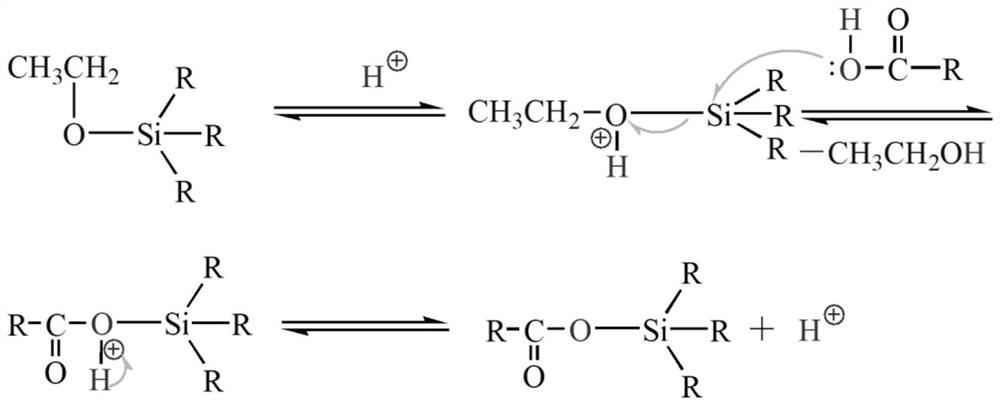
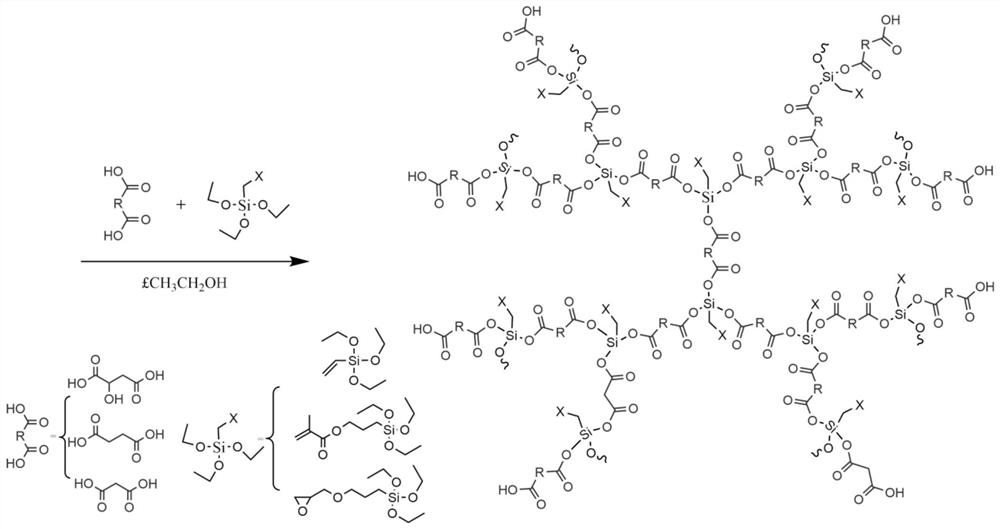
[0025] First, add malonic acid and methacryloxypropyltriethoxysilane into a three-necked round-bottomed flask at a molar ratio of 2:1, heat, feed nitrogen, stir, and slowly heat up to 85-130°C. When the system becomes clear and transparent, raise the temperature to 110-150°C. After a period of reaction, distillates will be produced, and continue the reaction until the distillation temperature drops below 30°C. When no distillates are produced, the reaction will stop and the reaction will be lowered to room temperature. , to obtain carboxy-terminated hyperbranched polysiloxane.
example 2
[0027] First, add malonic acid and 3-glycidyl etheroxypropyl triethoxysilane in a molar ratio of 2:1 into a three-necked round-bottomed flask, heat, blow in nitrogen, stir, and slowly heat up to 85-130°C , when the system becomes clear and transparent, the temperature is raised to 110-150°C. After a period of reaction, distillate is produced, and the reaction is continued until the distillation temperature drops below 30°C. When no distillate is produced, the reaction stops and drops to At room temperature, carboxyl-terminated hyperbranched polysiloxane can be obtained.
example 3
[0029] First, add succinic acid and vinyltriethoxysilane in a molar ratio of 2:1 into a three-necked round-bottomed flask, heat, blow in nitrogen, stir, and slowly heat up to 85-130°C, when the system becomes clear and transparent After that, the temperature is raised to 110-150°C. After a period of reaction, distillates are produced, and the reaction is continued until the distillation temperature drops below 30°C. When no distillate is produced, the reaction stops, and the carboxyl-sealed product can be obtained when the temperature is lowered to room temperature. terminal hyperbranched polysiloxane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com