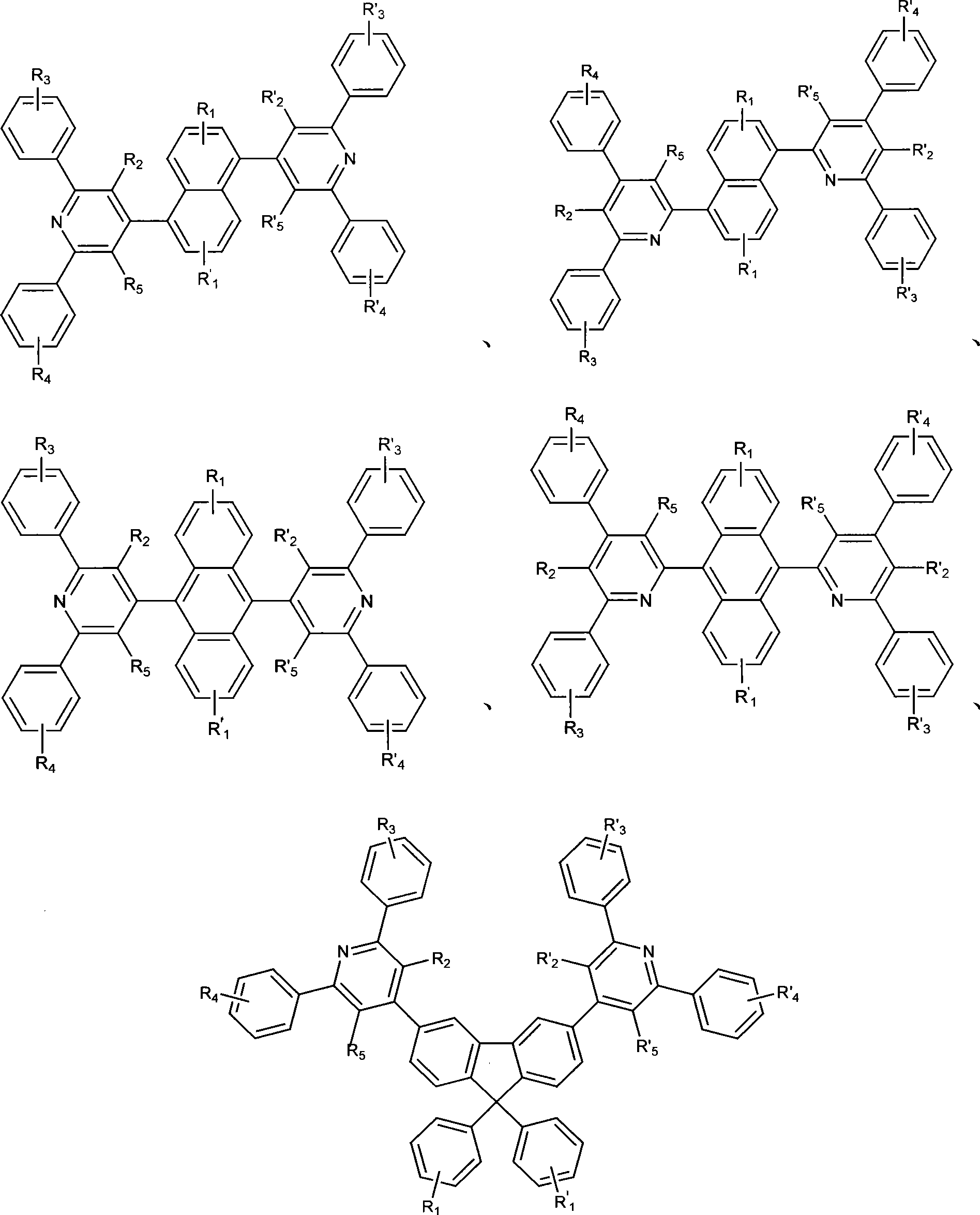
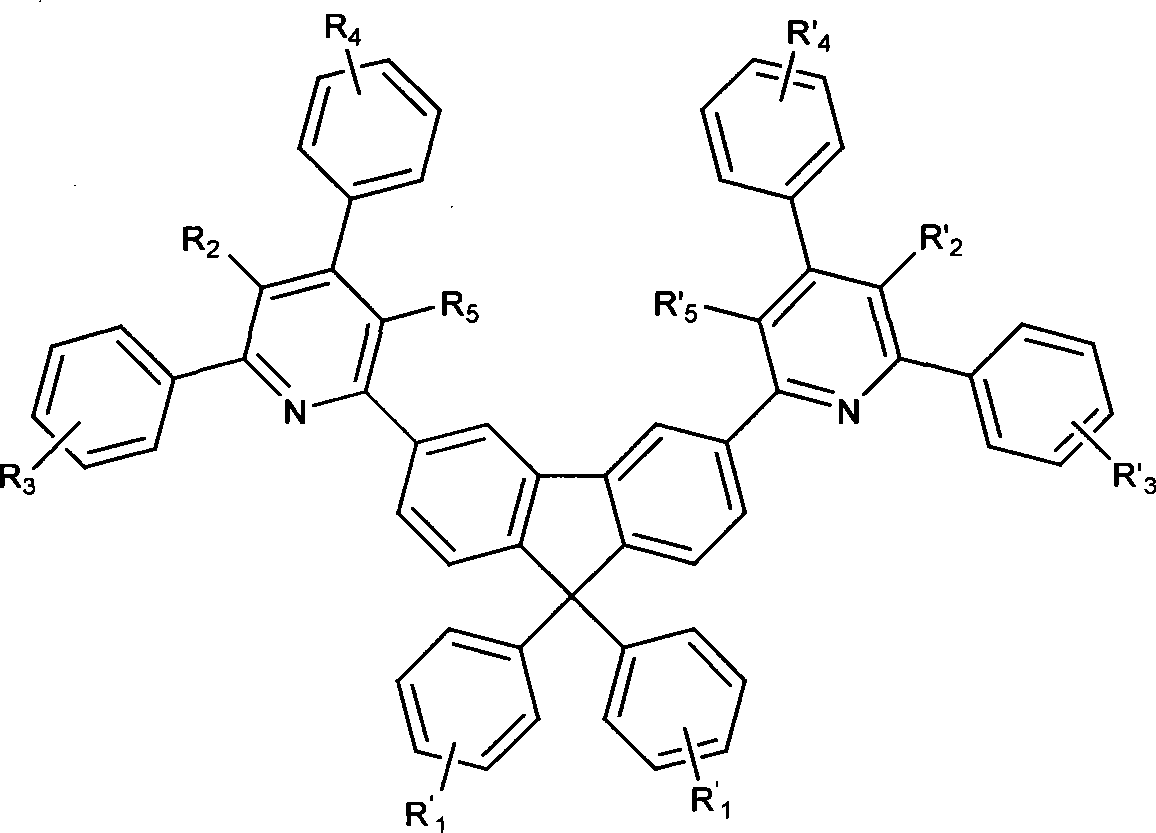
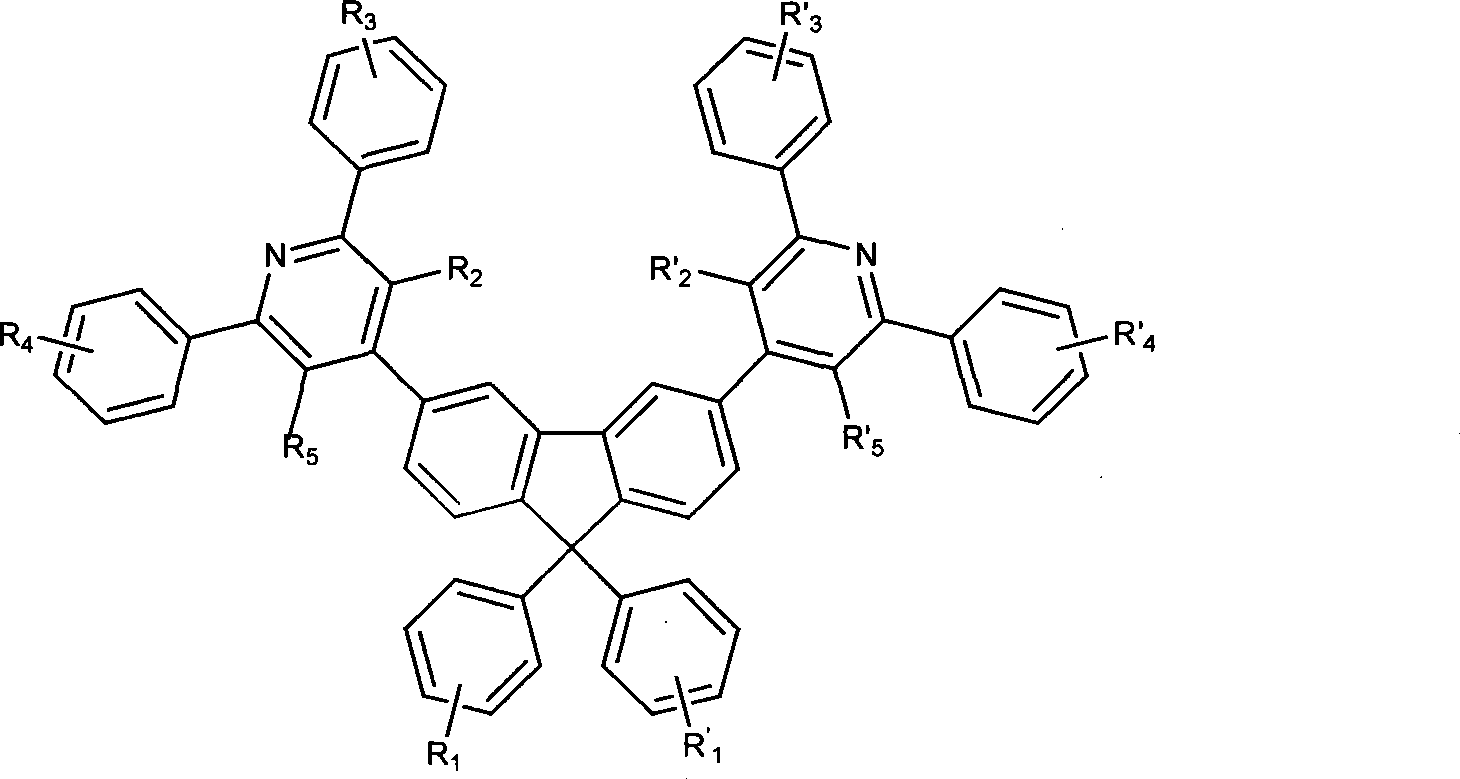
Electroluminescent organic material, synthetic method and use thereof
An electroluminescent material and electroluminescent technology, which can be used in luminescent materials, electroluminescent light sources, electric light sources, etc., and can solve problems such as poor device performance and changes in luminescent properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1.4,4'-(naphthyl-1,5-diyl) two (6-(4-tert-butylphenyl)-2-phenylpyridinecarbonitrile) (NBTNN) preparation
[0062]
[0063] The first step: get 4-(2-bromoacetyl) benzonitrile with a molar ratio of 1, pyridine is a raw material, stir at room temperature for 10 hours, filter, and wash with a large amount of water to obtain the corresponding pyridinium bromide, with a yield of about 85%;
[0064] The second step: under the condition of nitrogen protection, the first step product, 1,5-dialdehyde naphthalene and p-tert-butylacetophenone (molar ratio is 2:1:2) are added in the three-necked flask, and then Add an appropriate amount of glacial acetic acid and ammonium acetate, stir vigorously, keep the temperature at 120°C-140°C, reflux for 24 hours, filter the product, and obtain the high-purity target product through column chromatography or recrystallization, with a yield of about 55%. .
[0065] m / z: 748.36 (100.0%), 749.36 (58.9%), 750.36 (17.6%), 751.37 (3....
Embodiment 2
[0075] Example 2. Preparation of 1,5-bis(6-(4-tert-butylbenzene)-2-phenyl-3-trifluoromethylpyridin-4-yl)naphthalene (BBTPN)
[0076]
[0077] The first step: take 2-bromo-1-(4-trifluoromethylphenyl)ethanone and pyridine with a molar ratio of 1 as raw materials, stir at room temperature for 10 hours, filter, and wash with a large amount of water to obtain the corresponding pyridine Bromide, the yield is about 90%;
[0078] The second step: under the condition of nitrogen protection, the first step product, 1,5-dialdehyde naphthalene and p-tert-butylacetophenone (molar ratio is 2:1:2) are added in the three-necked flask, and then Add an appropriate amount of glacial acetic acid and ammonium acetate, stir vigorously, keep the temperature at 120°C-140°C, reflux for 24 hours, filter the product, and obtain the target product with high purity through column chromatography or recrystallization, with a yield of about 60%. .
[0079] m / z: 834.34 (100.0%), 835.34 (59.1%), 836.35 (1...
Embodiment 3
[0080] Embodiment 3.4, the preparation of 4'-(naphthyl-1,5-diyl) bis(6-(4-tert-butylphenyl)-2-phenylpyridine diethyl ester) (NBBNN)
[0081]
[0082] The first step: get 4-(2-bromoacetyl)ethyl benzoate with a molar ratio of 1, pyridine is a raw material, stir at room temperature for 9 hours, filter, and wash in a large amount of water to obtain the corresponding pyridinium bromide, producing The rate is about 80%;
[0083] The second step: under the condition of nitrogen protection, the first step product, 1,5-dialdehyde naphthalene and p-tert-butylacetophenone (molar ratio is 2:1:2) are added in the three-necked flask, and then Add an appropriate amount of glacial acetic acid and ammonium acetate, stir vigorously, keep the temperature at 120°C-140°C, reflux for 24 hours, filter the product, and obtain the target product with high purity through column chromatography or recrystallization, with a yield of about 50%. .
[0084] m / z: 842.41 (100.0%), 843.41 (64.2%), 844.42 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum brightness | aaaaa | aaaaa |
| Luminous efficiency | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com