Organic electronic transmission and/or hole barrier materials, synthesis method and use thereof
A technology of hole-blocking materials and organic electronics, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve problems such as changes in luminescent properties, stability to be further improved, and poor device performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
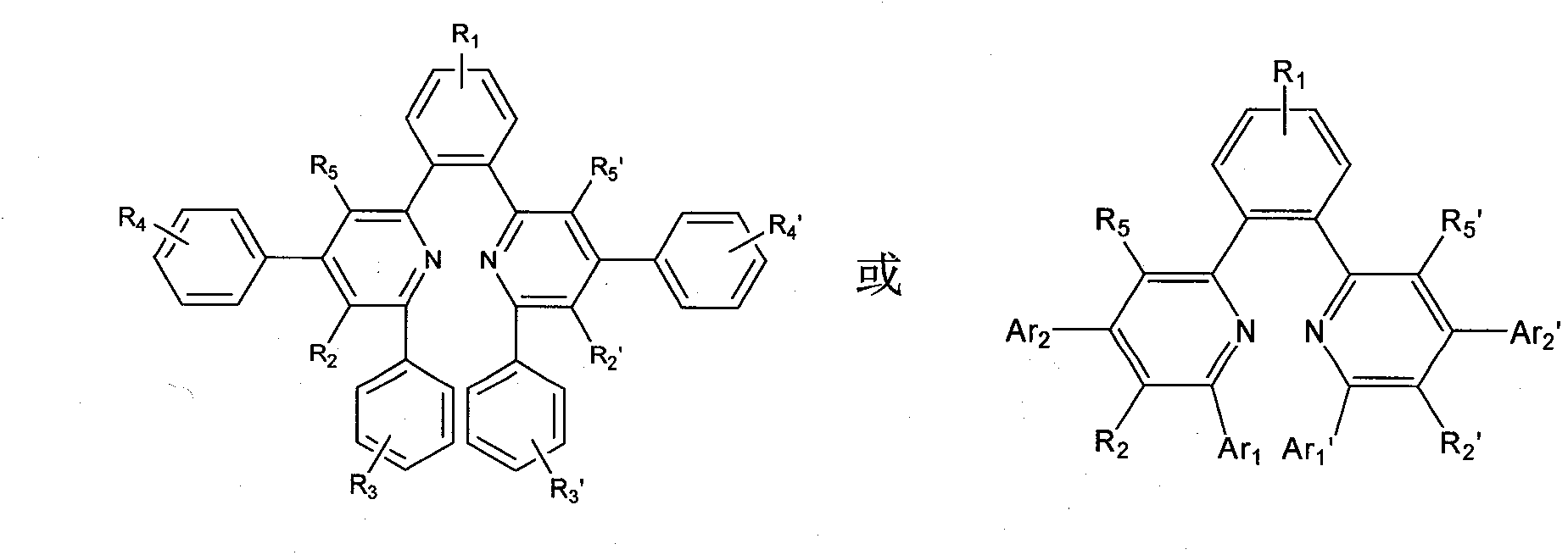
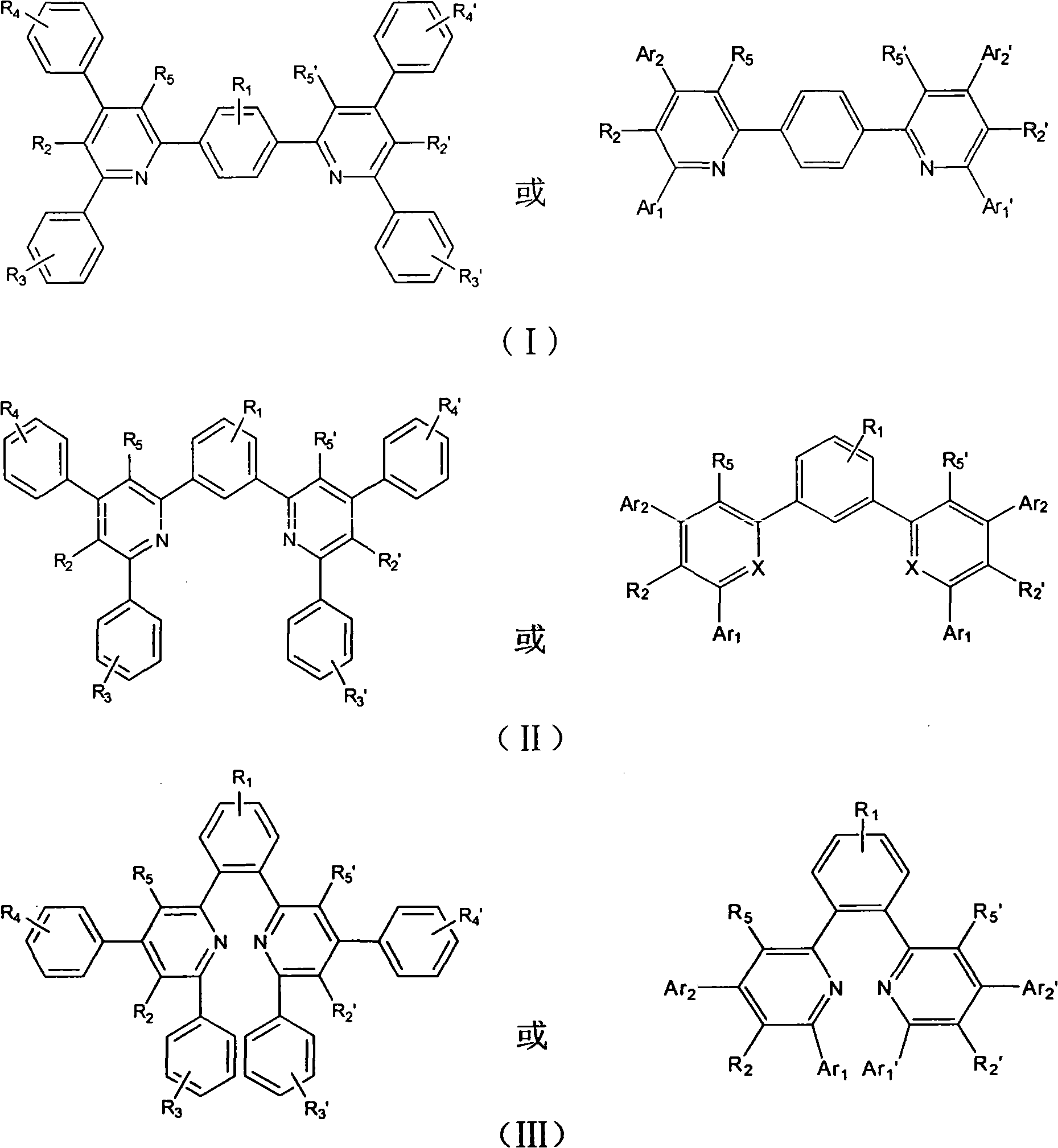
[0056] Embodiment 1.1, the preparation of 4-bis(2-(4,6-diphenyl-5-trifluoromethylpyridine))benzene (BDTPB)
[0057]
[0058] The first step: take 2-bromo-1-(4-trifluoromethylphenyl)ethanone and pyridine with a molar ratio of 1 as raw materials, stir at room temperature for 10 hours, filter, and wash with a large amount of water to obtain the corresponding pyridine Bromide, the yield is about 90%;
[0059] The second step: under the condition of nitrogen protection, add the product of the first step, p-benzophenone and benzaldehyde (2:1:2 in molar ratio) into the three-necked bottle, and then add appropriate amount of glacial acetic acid and ammonium acetate , stirred vigorously, kept the temperature at 120° C. to 140° C., refluxed for 24 hours, filtered out the product, and subjected to column chromatography or recrystallization to obtain the high-purity target product with a yield of about 50%.
[0060] m / z: 672.20 (100.0%), 673.20 (46.2%), 674.21 (10.2%), 675.21 (1.5%). ...
Embodiment 21
[0070] Example 2.1, Preparation of 4-bis(2-(6-phenyl-4-p-tolyl-5-trifluoromethylpyridine))benzene (BPTTPB)
[0071]
[0072] The first step: take 2-bromo-1-(4-trifluoromethylphenyl)ethanone and pyridine with a molar ratio of 1 as raw materials, stir at room temperature for 8 hours, filter, and wash with a large amount of water to obtain the corresponding pyridine Bromide, the yield is about 90%;
[0073] The second step: under the condition of nitrogen protection, the product of the first step, p-benzenediphenone and p-tolualdehyde (2:1:2 in molar ratio) are added in the three-necked flask, and then an appropriate amount of glacial acetic acid is added and ammonium acetate, stirred vigorously, kept the temperature at 120°C to 140°C, refluxed for 24 hours, filtered out the product, and subjected to column chromatography or recrystallization to obtain the high-purity target product with a yield of about 55%.
[0074] m / z: 700.23 (100.0%), 701.23 (48.3%), 702.24 (11.2%), 703....
Embodiment 36
[0075] Embodiment 3.6, the preparation of 6'-(1,4-phenylene) bis(2,4-diphenylpyridinenitrile) (PBDNN)
[0076]
[0077]The first step: take 4-(2-bromoacetyl) benzonitrile and pyridine with a molar ratio of 1 as raw materials, stir at room temperature for 7 hours, filter, and wash with a large amount of water to obtain the corresponding pyridinium bromide salt with a yield of about 85%;
[0078] The second step: under the condition of nitrogen protection, add the product of the first step, p-benzophenone and benzaldehyde (2:1:2 in molar ratio) into the three-necked bottle, and then add appropriate amount of glacial acetic acid and ammonium acetate , stirred vigorously, kept the temperature at 120° C. to 140° C., refluxed for 24 hours, filtered out the product, and subjected to column chromatography or recrystallization to obtain the high-purity target product with a yield of about 60%.
[0079] m / z: 586.22 (100.0%), 587.22 (45.7%), 588.22 (10.7%), 589.23 (1.5%), 587.21 (1.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com